Abstract
Granulocytic sarcoma is a localized extramedullary solid tumor composed of immature myeloid cell and is usually associated with acute myeloid leukemia or myelodysplastic syndrome. Although it can involve any site, commonly in lymph nodes, skin, bone and soft tissue, the involvement of breast is unusual. Especially, the involvement of the breast as a pattern of relapse after bone marrow transplantation is extremely rare. We have experienced 2 cases of granulocytic sarcoma after bone marrow transplantation. One case was a 39-year-old woman with right breast mass diagnosed with granulocytic sarcoma. She had received an unrelated bone marrow transplantation due to biphenotype acute leukemia 3 years before our presentation. Another case was a 48-year-old woman with acute myeloid leukemia, who was diagnosed with granulocytic sarcoma on both breasts 8 months after allogenic bone marrow transplantation. We also discuss the clinicopathologic features of granulocytic sarcoma in breast after bone marrow transplantation.
Granulocytic sarcoma is a rare extramedullary tumor composed of undifferentiated myeloid cells. It is known by various other names including monocytic sarcoma, extramedullary myeloid cell tumor, myeloblastoma, and chloroma. It was first reported by Burns in 1811 and was described as chloroma by King in 1853 due to the characteristic greenish colored tumor cells in myeloperoxidase staining. Later, Rappaport described it as granulocytic sarcoma in 1967 [1-3] and recently, this term has been deemed to be more appropriate, because all of the tumor cells are not stained greenish under such conditions. Granulocytic sarcoma usually occurs alongside with acute myeloid leukemia or myelodysplastic syndrome [1]. Granulocytic sarcoma can involve any site in the body and can present as multiple lesions. The most commonly involved sites include lymph node, skin, bone, central nervous system, and soft tissue. Rarely, it may occur as a mass of the breast [4-6]. We have experienced two cases of granulocytic sarcoma in breast and we report these rare cases.
A 39-year-old woman was admitted to our breast clinic with a palpable mass in the right breast that had been increasing in size for the past four months. Upon physical examination, a fixed and firm mass was palpated on the right breast. There were no signs of nipple retraction or skin abnormalities. Breast ultrasonography demonstrated multiple masses on the right breast with enlarged axillary lymph node (Figure 1). Core needle biopsy was performed and atypical mononuclear hematopoietic cells were identified along with diffuse periductal infiltration of hyperchromatic cells which had a large nuclear-cytoplasmic ratio. The results of immunohistochemistry were positive for cluster of differentiation (CD)34, lysozyme, and myeloperoxidase (MPO) and were negative for CD3 and CD20. We could conclude granulocytic sarcoma by these immunohistochemical findings.
The patient had an admission history for petechiae and easy bruises along the whole body 4 years previously. The thrombocytopenia and anemia were identified (platelet count 16,000/µL, hemoglobin 8.6 g/dL, hematocrit 23.9%) and 21% of blasts were observed by peripheral blood smear. In suspicion of acute leukemia, she was referred to the Department of Hematology-Oncology and received bone marrow examination. The blast cells in the bone marrow were infiltrated diffusely and cellularity was increased up to 95%. The results of immunophenotyping were positive for human leukocyte antigen (HLA)-DR (39%), CD10 (31%), CD19 (43%), CD22 (36%), CD3 (34%), CD5 (33%), CD7 (34%), CD33 (27%), and CD34 (52%), which was in accordance with biphenotype acute leukemia (acute lymphoid leukemia > acute myeloid leukemia). She received remission induction therapy (cytarabine 200 mg/m2/day for 7 days, daunorubicin 60 mg/m2/day for 3 days) and then consolidation therapy (cytarabine 2 g/m2/day for 4 days, daunorubicin 45 mg/m2/day for 3 days). She eventually received an unrelated bone marrow transplantation. During following observation, she visited our breast clinic for a palpable mass in the right breast and was diagnosed with granulocytic sarcoma. We recommended chemotherapy but the treatment was delayed because of her financial circumstances. She was admitted with a headache 2 months later. Multiple brain metastases were identified by computed tomography (CT). She underwent salvage chemotherapy (imatinib 400 mg and hyperCVAD: cyclophosphamide 300 mg/m2/day every 12 hours for 3 days, vincristine 2 mg/day for 2 days, doxorubicine 50 mg/m2/day for 1 day, dexamethasone 40 mg/day for 8 days) but the disease progressed rapidly and she expired 2 months after salvage chemotherapy.
A 48-year-old woman was admitted to our breast clinic for palpable mass on each breast that had been increasing in size for the previous 2 weeks. Fixed and firm masses were palpable on both breasts by physical examination at the time of her visit. Mammography showed multiple masses on both breasts and bilateral enlargement of axillary lymph nodes (Figure 2A and B). Sonographic images showed multiple heterogeneous hypoechoic masses with echogenic boundaries at both breast (Figure 2C and D). Breast magnetic resonance imaging (MRI) showed contrast enhancement of multiple masses on both breasts with central necrotic lesions, suggestive of malignant tumors (Figure 2E and F). Gun biopsy was performed on her breast mass. The periductal parenchymal hyperchromatic cells were infiltrated diffusely (Figure 3A). Immunohistochemical staining was performed in order to rule out other malignant tumors and the results were positive for CD34, CD43, and MPO and negative for CD3 and CD20. These findings were matched with granulocytic sarcoma (Figure 3B).
The patient had a past history of visiting a local obstetrics-gynecology for hypermenorrhea. White blood cell count was 169,800/µL, hemoglobin was 7.7 g/dL, and platelet count was 12,000/µL. Ninety-two percent of blast cells were observed by peripheral blood smear. She was referred to the Department of Hematology-Oncology. Abnormal blast cells in bone marrow were infiltrated diffusely and cellularity was increased to more than 90% by bone marrow examination. The results of bone marrow aspiration biopsy were positive for CD7, CD13, CD33, CD34, and HLA-DR. She was diagnosed with acute myeloid leukemia (M1) and received remission induction therapy (cytarabine 200 mg/m2/day for 7 days, idarubicin 12 mg/m2/day for 3 days), consolidation therapy (danorubicin 45 mg/m2/day for 3 days, cytarabine 2 g/m2/day for 4 days), and allogenic hematopoietic stem cell transplantation. During following observation, she visited our breast clinic for palpable masses that were increasing in size. There was incomplete remission after starting second-line remission induction therapy (MEC543: mitoxantrone 10 mg/m2/day for 3 days, etoposide 100 mg/m2/day for 4 days, and cytarabine 2,000 mg/m2/day for 5 days) and almost complete clinical remission after receiving radiation therapy at the remaining breast tumor for 2 months. However, the tumor metastasized to ear, skin, and ovaries and she eventually expired 2 years after diagnosis of granulocytic sarcoma.
Granulocytic sarcoma is an extramedullary tumor composed of myeloid progenitor cells. It is also known as chloroma because it contains myeloperoxidase which is responsible for its green-colored appearance [2]. Granulocytic sarcoma can be classified into three clinically distinct occurrences. The first category may occur primarily as an extramedullary lesion before the occurrence of hematologic diseases such as acute leukemia. The second category may occur at the time of diagnosis of acute leukemia. The third category may occur when myelodysplastic syndrome progresses to leukemia or when erythroleukemic blast crisis occurs from chronic myeloid leukemia [6]. The involvement of the breast is very rare [5]. Viadana et al. [7] examined metastatic lesions through autopsy of 503 leukemia patients, and reported only 4 (1.7%) cases of breast invasion among 235 acute myeloid leukemia patients. Liu et al. [8] also performed autopsy of 237 acute myeloid leukemia patients and reported only 2 (0.8%) cases of metastatic breast tumor. In South Korea, Joo et al. [9] reported a case of a 42-year-old woman with granulocytic sarcoma on the breast before the diagnosis of leukemia. Park et al. [10] reported the case of a 49-year-old woman, who had gained complete remission after receiving remission induction therapy, and had a recurrence of granulocytic sarcoma on the breast prior to bone marrow recurrence. This differs to our case in that the granulocytic sarcoma occurred prior to bone marrow transplantation. In our case, they had already received bone marrow transplantation after achieving complete remission via remission induction and consolidation therapy and breast was the first site of relapse. Extramedullary recurrence is rare in acute myeloid leukemia patients who have received bone marrow transplantation. Békássy et al. [11] reported only 20 (0.65%) cases of extramedullary recurrence among 3,071 acute myeloid leukemia patients who have received bone marrow transplantation.
Granulocytic sarcoma is usually large and multiple masses with rapid growth. On MRI, it may show peripheral enhancement of central necrotic lesion. On mammography or breast ultrasonography, it may be difficult to differentiate with other benign or malignant neoplasm [12]. Granulocytic sarcoma of the breast should be differentiated with large cell non-Hodgkin's lymphoma, malignant melanoma, neuroendocrine tumor, undifferentiated carcinoma including invasive small cell carcinoma, extramedullary hematopoiesis, and infection [5,9,13]. Immunophenotyping after biopsy is essential for differential diagnosis. CD68 (KP1) and MPO are high in sensitivity and specificity for diagnosis of granulocytic sarcoma [6,13]. Therefore, even though rare, biopsy of breast mass and immunohistochemistry must be performed to rule out possibilities of granulocytic sarcoma in leukemic patients.
Prognosis of granulocytic sarcoma varies with clinical situations [3,7,14]. In chronic myeloid leukemia, the prognosis is poor because granulocytic sarcoma represents progression into erythroleukemic blast crisis or acute myeloid leukemia. However, the prognosis is not affected in acute myeloid leukemia patients. Recurrence after bone marrow transplantation is related to poor prognosis since recurrence reflects resistance or failure to therapy [11]. Depending on the clinical situation, the treatment for granulocytic sarcoma may vary from chemotherapy to radiotherapy. Furthermore, aggressive treatment including bone marrow transplantation is recommended [6,15]. Although only few data are available on this unusual disease, accurate diagnosis is required to avoid unnecessary surgery and early aggressive antileukemic therapy.
Figures and Tables
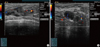 | Figure 1Case 1. Ultrasonographic images of right breast (A) and axilla (B). Heterogenous hypoechoic masses with multifocal cystic portion in entire right breast with right axillary lymphadenopathy were identified. |
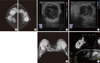 | Figure 2Case 2. Radiologic findings of the breast. (A, B) Mammograms show multiple various sized circumscribed and ill defined iso to hypodense masses at both breast (A, right craniocaudal view; B, left craniocaudal view). (C, D) Sonographic images show multiple heterogeneous hypoechoic masses with echogenic boundary at both breast (C, right; D, left). (E, F) Magnetic resonance imaging findings show multiple variable sized enhancing masses in both breasts (E) and most of them show internal necrotic portion (F). |
References
1. Neiman RS, Barcos M, Berard C, Bonner H, Mann R, Rydell RE, et al. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer. 1981. 48:1426–1437.

3. Baer MR. Management of unusual presentations of acute leukemia. Hematol Oncol Clin North Am. 1993. 7:275–292.

4. Breccia M, Mandelli F, Petti MC, D'Andrea M, Pescarmona E, Pileri SA, et al. Clinico-pathological characteristics of myeloid sarcoma at diagnosis and during follow-up: report of 12 cases from a single institution. Leuk Res. 2004. 28:1165–1169.

5. Ngu IW, Sinclair EC, Greenaway S, Greenberg ML. Unusual presentation of granulocytic sarcoma in the breast: a case report and review of the literature. Diagn Cytopathol. 2001. 24:53–57.

6. Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007. 21:340–350.

7. Viadana E, Bross ID, Pickren JW. An autopsy study of the metastatic patterns of human leukemias. Oncology. 1978. 35:87–96.

8. Liu PI, Ishimaru T, McGregor DH, Okada H, Steer A. Autopsy study of granulocytic sarcoma (chloroma) in patients with myelogenous leukemia, Hiroshima-Nagasaki 1949-1969. Cancer. 1973. 31:948–955.

9. Joo M, Lee HK, Kang YK, Kim JH. Granulocytic sarcoma of the breast preceding acute myelogenous leukemia: a case report. J Korean Med Sci. 2000. 15:457–459.

10. Park SK, Suh SH, Nam HK, Kang AY, Kim DC, Han JY, et al. A case of granulocytic sarcoma relapsed in both breasts after complete remission of acute myelogeous leukemia. Korean J Lab Med. 2005. 25:223–226.
11. Békássy AN, Hermans J, Gorin NC, Gratwohl A. Granulocytic sarcoma after allogeneic bone marrow transplantation: a retrospective European multicenter survey. Acute and Chronic Leukemia Working Parties of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1996. 17:801–808.
12. Basara I, Orguc S. Giant breast involvement in acute lymphoblastic leukemia: MRI findings. J Breast Cancer. 2012. 15:258–260.

13. Markoc F, Bozdogan N, Yükrük FA, Gumuc EB, Akdur NC. Granulocytic sarcomas: difficulties in diagnosis. Tumori. 2010. 96:149–153.

14. Chua ET. A case of granulocytic sarcoma of the breast and review of the literature. Singapore Med J. 1989. 30:311–312.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download