Abstract
Breast cancer cure aims at complete elimination of malignant cells and essentially requires detection and treatment of any micrometastases. Here, we present a review of the current methods in use and the potential role of the quantum dots (QDs) in detection and visualization of sentinel lymph node and micrometastases in breast cancer patients. The traditional histopathological, immunohistochemical, and reverse transcriptase polymerase chain reaction procedures being used for micrometastases detection had serious drawbacks of high false negativity, specificity variations and false positivity of the results. Photon emission fluorescence multiplexing characteristics of the quantum dots make them potentially ideal probes for studying the dynamics of cellular processes over time such as continuous tracking of cell migration, differentiation, and metastases. In breast cancer, QDs based molecular and genomic detections had an unparallel high sensitivity and specificity.
The aims of modern breast cancer surgery are to obtain local and regional control of the cancer and gather sufficient evidence to detect/predict the risk of distant metastases in order to guide systemic therapy. In breast cancer, this has traditionally been achieved by resection of the primary tumor (either by mastectomy or by wide local excision) and axillary lymph node dissection (ALND). ALND, however, has significant short- and long-term morbidity, with widespread use of breast-conserving surgery, the staging ALND procedure carries greater morbidity than the therapeutic procedure of the primary cancer. Sentinel lymph node (SLN) biopsy is a minimally invasive technique to stage the axilla in breast cancer, without compromising the prognostic information obtained from ALND. Studies comparing ALND and SLN biopsy have demonstrated that SLN biopsy is an accurate staging technique, which results in a significant reduction of ALND associated morbidity [1].
Metastatic breast cancer is characterized by spreading of cancer cells into the nearby breast tissue, and other body parts (e.g., bone, lymph nodes, liver, and lungs), and metastasis is the main cause of death in breast cancer patients. As in many other metastases prone cancers, specific molecular changes occurring within the tumor cells and tumor microenvironment contribute to the detachment of tumor cells from the primary tumor mass, invasion into the tumor stroma, intravasation into nearby blood vessels or lymphatics, survival in the blood stream, extravasation into and micrometastases (MMs) development in the target organ, and finally, metastatic outgrowth [2]. Complete resection of the primary tumor, SLNs, likely containing tumor-cells and any small adjacent satellite nodules in the absence of distant metastases, is associated with three to five fold improvements in patient's survival, as compared to a partial or incomplete resection [3-6]. Earlier studies on the subject suggest that at the time of diagnosis, 35% to 40% of the breast cancer patients already have occult metastases in the bone and lymph nodes (called disseminated tumor cells [DTCs]) and their counterparts in circulation known as circulating tumor cells (CTCs) which after the resection of primary tumor often progress to micrometastatic, and later metastatic disease. There are a few evidences that favor the possibility that these DTCs/CTCs give rise to metastases. First, the presence of DTCs in bone marrow is significantly correlated with metastatic relapse. Second, most DTCs/CTCs are nonproliferating (that is, Ki-67 negative) and resistant to chemotherapy, and they can persist in the bone marrow of patients with breast cancer many years after primary surgery. Third, DTCs/CTCs have a breast cancer stem-cell phenotype (for example, CD44+, CD24-/low, cytokeratin 19+, MUC1-, EpCAM+). Fourth, the two stem cell factors epidermal growth factor (EGF) and fibroblast growth factor 2, are required for the in vitro growth of DTCs obtained from the bone marrow of patients with cancer. Nevertheless, strong direct evidence that some of the DTCs or CTCs detected in bone marrow or blood samples have cancer stem cell properties is still lacking [7,8]. Some parameters as tumor size, lymph node status and histological sub-type are traditionally used for the risk assessment of recurrence, and needs relapse-preventive adjuvant anticancer therapy. With these current tools, however, the presence of minimal residual disease is only presumed rather than measured, for which the occult metastases may likely lead to failure of the primary treatment. Also, the presently used chemotherapeutic drugs being aimed at the proliferating cells cannot target the dormant sub-type of cancer cells and may even increase the micrometastatic disease [9,10]. The occult micrometastatic deposits, thus, remain the untreated source of potential subsequent relapses. The occult residual metastases have recently been divided into two categories: First, micrometastases, comprising of tumor deposits of 0.2 to 2 mm size. Second, isolated tumor cells (ITCs) of <0.2 mm in size [11]. Distinction between ITCs and micrometastases is necessary as it influences treatment decision. Micrometastases are considered true nodal metastases, requiring treatment by dissection and adjuvant chemotherapy. The ITCs are not considered significant enough to categorize them as a tumor, and are regarded as node-negative for both the staging and treatment decisions [12-14]. Hence, besides the resection of primary tumor, a complete cancer cure essentially requires detection and treatment of any nodal micrometastases. Here, we present review of the traditional methods in use and the potential role of quantum dots in detection of SLN micrometastases.
The sentinel node is defined as any lymph node(s) receiving direct lymphatic drainage from the primary tumor, and therefore is the first node to become involved when a tumor metastasizes via lymphatics. The concept behind SLN biopsy is that lymphatic metastases occur in an orderly manner and the status of sentinel node predicts the status of other regional lymph nodes. If the sentinel node does not contain any metastatic cells, the draining nodal basin is highly unlikely to harbor metastases and complete nodal dissection is not required. SLN mapping can be performed by a variety of imaging methods, including blue dye injection, nuclear lymphoscintigraphy, computed tomography (CT), and magnetic resonance imaging (MRI). However, each of these imaging methods have their own limitations: the blue-dye injection method is less sensitive to subsurface tissue and has almost no tissue penetration, lymphoscintigraphy has low spatial resolution and requires exposure to radioactivity, and CT/MRI requires a bulky imaging machine that prevents their use during surgery. Advantages of optical imaging with quantum dots (QDs) are that it can be performed without ionizing radiation with sufficient spatial resolution in real time during surgery [15]. Although patients with node-negative breast cancer have an excellent prognosis, up to 25% to 30% of these patients will develop local recurrences or distant metastases within 10 years [16]. Studies have suggested that this unfavorable outcome may be due in part to undetected occult metastases in the lymph nodes.
Histopathological examination of the SLN for micrometastases detection has a major drawback of false negative reporting [17]. Since, on the routine tissue sections the pathologist has only 1% chance of identifying a micrometastatic focus of the diameter of three cells in cross section [18]. It implies that micrometastases consisting up to three cells when present in the section has 99% possibility of being missed in the diagnosis [19]. Histopathology of the sentinel and regional lymph nodes is not a reliable method for detection of micrometastases, but because of low cost, easy availability, and simple procedure, it is still being used worldwide.
IHC staining using various monoclonal and polyclonal antibodies against the epithelial and other antigens expressed on the cancer cells has been developed to identify breast cancer micrometastases/metastases in the excised tissues [20-22]. Anti-CK antibodies are the most commonly used agents for micrometastases detection because their sensitivity and specificity is higher compared to other anti-epithelium-specific antibodies. Other antigens for micrometastases detection of breast cancer are anti-human epidermal growth factor receptors (HER)2, anti-epithelial cell adhesion molecule (anti-EpCAM), and anti-MUC1 [23]. Although IHC staining for cytokeratin is not routinely advocated by any consensus recommendation, it is commonly used in routine practice in the United States and in many European countries [24-27]. SLN cytokeratin and epithelial membrane antigen IHC screening reported micrometastases in 9% to 30% of those cases, in which the lymph nodes were earlier declared negative on histopathology [28-30]. IHC is especially helpful for detection of micrometastases in those histopathology negative cases, where cancer cells do not form a cohesive cluster and lie scattered within the lymph node. If the number of these scattered cancer cells decrease further their chances to get detected declines even on IHC. Although IHC is sensitive for micrometastases detection, but, there are certain disadvantages, like-loss of cytokeratin expression may occur in cancer cells, tumor antigens related specificity variations, high tissue auto fluorescence, reduction of antigen during fixation and embedding, subjective variations in interpretation of result, and time consumption [30-33].
In the last decade, molecular diagnosis of breast cancer micrometastases has been under focus using RT-PCR by detecting messenger RNA related to the breast carcinoma cells. It could detect 1 metastatic cell among 106 normal lymphoid cells of the node. The markers used in this technique included cytokeratins, MUC-1, mammaglobin, and carcinoembryonic antigen. From 15% to 40% of lymph nodes reported negative for micrometestases on histopathology and IHC turned out to be positive with RT-PCR [34-38]. However, RT-PCR is so sensitive that false positivity, due to contamination by normal breast cells causing epithelial or tissue related transcription has been a serious concern. In addition, cross-reactivity with homologous genes may lead to further false-positive results [39,40]. Moreover, heterogeneity in the expression levels of a particular target transcript between individual micrometastatic cells cannot be predicted by RT-PCR.
Fluorescent imaging has emerged with some unique capabilities for molecular cancer imaging. Fluorescence is the light emitted by certain molecules when they absorb light at a shorter wavelength (excitation). These fluorophores emit light throughout the visible spectrum, however, the best spectrum for in vivo imaging is in the near-infrared (NIR) region (650-900 nm) [41]. Unlike the visible light spectrum (400-650 nm), in the NIR region, light scattering decreases and photo absorption by hemoglobin and water diminishes, leading to deeper tissue penetration of light. Furthermore, tissue autofluorescence is low in the NIR spectra. QDs are nearly spherical crystalline semiconductor particles, less than 10 nm in diameter, containing roughly 200 to 1,000 atoms [42]. "Quantum" signifies the fact that their behavior and properties are governed to a significant extent by quantum mechanics, rather than classical mechanics and "dot" corresponds to the extremely small dimension of these particles. The majority of QDs are binary semiconductor crystals, composed of two types of atoms from the II/VI (cadmium selenide [CdSe]) or III/V (InP) group elements of the periodic table. In general, each QD is composed of a binary crystal core and capped with a binary shell that stabilizes and increases its quantum yield. These semiconductors are characterized by composition dependent band gap energy. The band gap energy is the minimal energy required to excite an electron from its ground state to a higher level. As the electron relaxes and returns to the ground state, a photon is emitted, leading to visible fluorescence. The band gap energy is dependent on the size of the semiconductor particle, larger QDs have smaller band gaps, resulting in emission of low energy red light, while smaller QDs emit blue light of higher energy. Furthermore, due to their small size, the entire particle acts as a single molecule with all constituent atoms exciting and emitting light simultaneously producing high signal intensity leading to an extremely high fluorescence. The optical characteristics of QDs can thus be tuned by adjusting their size [43]. Increasing size also improves penetration depth and reduces background fluorescence at the NIR wavelengths. The surface of these QDs is passivated with a monolayer of organic solvent, such as tri-n-octylphosphine oxide (TOPO). TOPO passivated QDs are hydrophobic and insoluble in aqueous solutions. To achieve water solubility and biocompatibility, QDs are often encapsulated with various types of amphiphilic polymers, which increase their hydrodynamic radius to about 20 nm. These amphiphilic polymers often carry chemically reactive groups, such as amines and carboxylic acids, which allow conjugation with biomolecules, such as peptides, proteins, and nucleic acids. Encapsulation and bioconjugation do not usually alter the optical properties of QDs significantly. Cd/Se QDs are the most studied, but they are not very suitable for in vivo imaging, due to their short emission wavelength (470-655 nm) that gets absorbed by tissue chromophores. The so-called types II QDs emit fluorescence in the far-red and NIR range, which makes them ideal for in vivo cancer imaging, since tissue chromophores in the epidermis and hairs absorb light in the infrared range wavelengths weakly. A type II QD consists of a Cd/Te core with a Cd/Se (or ZnS) shell; the increased thickness of their shell correlates with their higher emission wavelength in vivo.
They also display greater resistance against metabolic degradation and photobleaching (due to their inorganic composition) enabling investigations to be carried out over time. In addition, simultaneous excitation of multiple florescence colors via a single light source is possible [44]. These advantages make them ideal for the investigation of dynamics of cellular processes over time, such as continuously tracking cell migration, differentiation, and metastases. The long excited state lifetime of QDs also provide means for differentiation of the QD fluorescence signal from background fluorescence [45]. QDs have composition and size-dependent absorption and emission, and their size and shape can be controlled in a precise manner by time, temperature and ligand molecules during their synthesis [46]. This allows the synthesis of most of the highly luminescent QDs with many colors-this property represents a very promising advantage of QDs since cancer involves a number of genes and proteins. Tracking a panel of molecular markers at the same time will lead to better understanding, classifying and differentiating cancer than a single biomarker each time. QDs multiplexing also provide unique opportunities for simultaneous imaging of different targets using NIR wavelengths for SLN mapping [47]. Compared with organic dyes and fluorescent proteins, QDs have several advantages and unique applications. Firstly, QDs have very large molar extinction coefficients, about 10 to 50 times larger than that of organic dyes. Therefore, QDs are able to absorb 10 to 50 times more photons than organic dyes at the same excitation photon flux, leading to a significant improvement in the probe brightness. Secondly, QDs are several thousand times more resistant against photobleaching than organic dyes and are well-suited for studies over a long period of time. Thirdly, the large Stokes shifts of QDs (measured by the distance between the excitation and emission peaks) can be used to further improve the detection sensitivity. Organic dye signals with a small Stokes shift are often buried by strong tissue autofluorescence, whereas QD signals with a large Stokes shift are clearly detectable above the background [48]. Owing to the advantages mentioned above, QDs have utility in bioimaging, real-time fluorescence tracking and detection of live cells in vivo, diagnostic assays for cancer detection, detecting local extension of cancer cells and their distant metastases, and identifying the surgical tumor margins on the operation table.
One of the most promising and rapidly developing areas of application of QDs is their usage as fluorescent labels during in vitro study of tumor cells: for imaging tumor cells and localizing the individual molecules expressed in them. In vitro diagnostics, is the only application of QDs out of all other alternatives available, which can be quickly implemented in clinical practice (as opposed to the in vivo use of QDs, which requires long investigations of QD toxicity and further consequences of their introduction into the body of research organism). The major utilities of QDs as an in vitro diagnostic tool are: imaging of tumor cells overexpressing certain oncomarkers, staining of tissues and their sections, and observation of individual molecules and cells in real time.
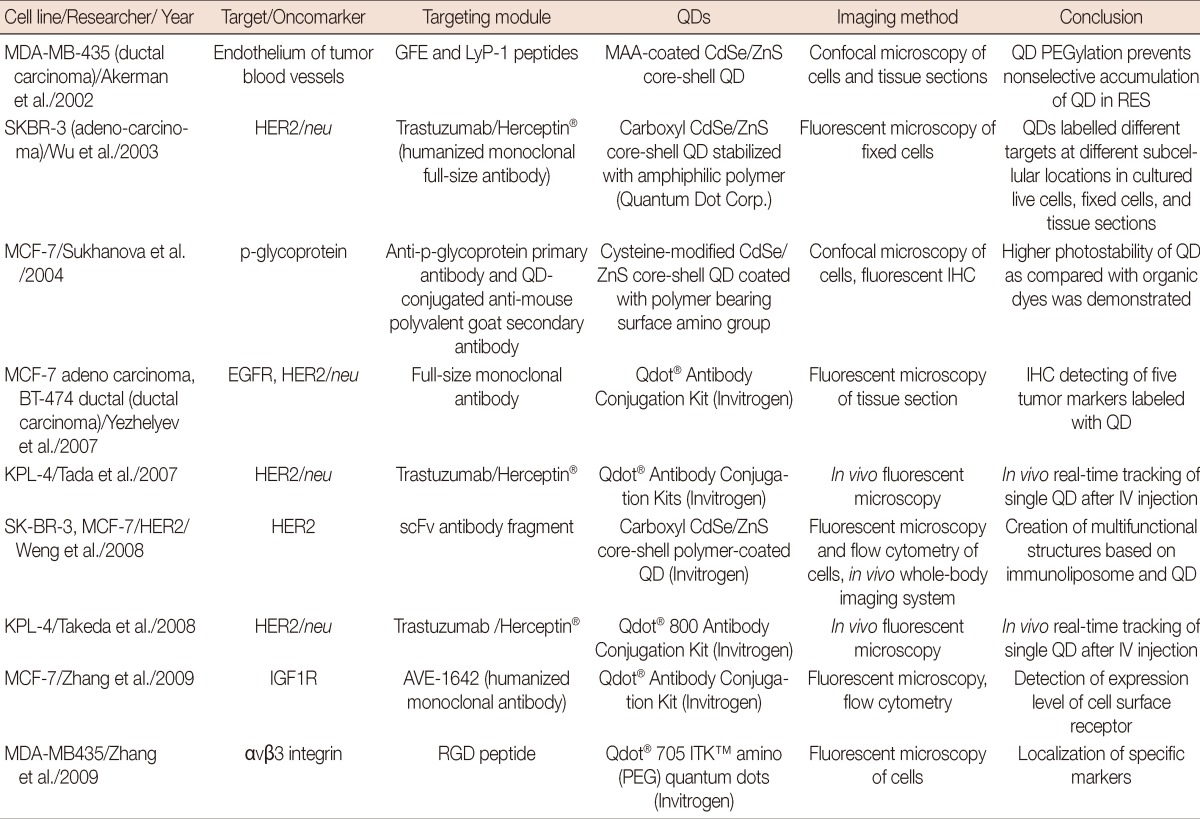
The imaging of tumor cells and identification of the individual oncomarkers and their quantitative assessment is very important for the early diagnosis, accurate classification, and optimal therapy of tumors. QDs that conjugated to various targeting agents (antibodies, ligands, peptides) have been known. They are intended for visualizing the cells of clinically significant human tumors: prostate carcinoma, breast adenocarcinoma and ductal carcinoma, pancreatic carcinoma, glioblastoma and squamous cell carcinoma of the tongue. Over a short period of time, appreciably simple, inexpensive, and well reproducible QD-based methods for the imaging of cancer cells were designed to aid in the diagnosis and predicting prognosis of cancers, especially breast cancer (Table 1) [48].
The surface area of nanocrystal QDs is appreciably large and is accessible for binding of several biological molecules. A range of 2 to 5 protein molecules and more than 50 small molecules (oligonucleotides or peptides) can be bound to one nanoparticle 4 nm in diameter. Targeting molecules (antigens, oligonucleotides, peptides, aptamers, etc.) that selectively bind to the surface oncomarker provide a high specificity for labeling the corresponding tumor cells. At the same time, such a feature of tumor cells as their extreme variability during the development of cancer and response to the action of therapeutic agents raises for researchers the problem of simultaneous imaging of several surface markers. The fundamental possibility of using QDs for simultaneous multiplex detection was demonstrated on five tumor markers from a human breast tumor cell culture. The simultaneous detection of the receptors estrogen receptor, progesterone receptor, EGF receptor, mTOP, and HER2/neu, using QDs fluorescence in different spectrum regions correlates positively with the results of conventional methods; including immunohistochemistry, Western blotting, and fluorescence in situ hybridization (FISH), while it considerably increases the rate of analysis and reduces cost [49]. Simultaneous imaging of two hypothesized cancer markers-integrin αvβ3 and nucleolin-using QDs conjugated to the RGD peptide and aptamer AS1411, respectively, enables to compare the localization of these markers within the cell [50]. Internalization of nucleolin and the surface distribution of integrin were confirmed using confocal microscopy, which will probably allow better understanding of how they participate in the processes occurring in tumor cells.
The results of these studies demonstrate that QDs conjugated to targeting molecules have a powerful potential as components of novel systems for assessing tumor types, their progression stage, and the metastatic potential on the basis of multiplex imaging. The combination of conventional IHC procedures with QD-based fluorescent dyes allows considerable improvement in the resolution and sensitivity of the method and provides possibility of simultaneous imaging of several markers. The feature of multiplexing is becoming increasingly important for cancer diagnosis since more and more studies are showing that a panel of biomarkers rather than a single one is needed to accurately determine the stage of the disease. Multiplexing has the advantage of reducing variability between tissue slices and is especially appealing in the case of precious specimens. Moreover the conjugation of QDs with the IHC method makes it much more illustrative. The biomarkers used to detect breast cancer micrometastases in lymph node like: anti-CK antibodies, anti-HER2 antibodies, anti-EpCAM, and anti-MUC1 can be detected by the combined QD-IHC approach, with results much superior to conventional IHC, and sensitivity nearly equal to that of FISH. As for example, HER2 status is usually assessed by standard IHC, with additional FISH in ambiguous cases. However, FISH is expensive, labor intensive and time consuming. Streptavidin coated QDs were used to visualize HER2 positivity after immunohistochemical staining with primary and secondary anti-HER2 antibodies and fluorescence image analysis used to identify and quantify positive signals. The results of QD-IHC were compared to both FISH analysis and conventional IHC in the same tissue samples. Good concordance between QD-IHC and FISH results was seen. The results indicate the feasibility and utility of quantitative IHC using QDs, this approach may be particularly useful in developing countries, where access to molecular techniques, such as FISH, is low [51]. Similarly, other biomarkers of micrometastases can also be detected with a combined QD-IHC approach.
Cell dynamics, molecular signaling mechanisms, conformational changes of proteins etc. are crucial in understanding the processes like chemotaxis and intercellular and intracellular signal transduction. In order to use QDs to gather information about specific cellular processes in real time, a specific cellular target must be considered QDs conjugated with the corresponding targeting ligands have been successfully used for such imaging modalities. Since a vast number of oncomarkers are represented by proteins that also have some regulatory functions in the normal cells and participate in various inter- and intracellular signal transductions.
Studying in detail the functioning of these oncomarkers/proteins is important to understand the multiple steps and mechanisms leading to malignant transformation of a normal cell. QDs conjugated with an EGF were used to study the mechanisms of EGF internalization and signal transduction pathways with the participation of proteins from the erbB-1/2/3 family of transmembrane tyrosine kinase receptors [52]. A study in breast cancer used QDs linked to streptavidin, which allowed them to bind with biotin conjugated targeting agents.
Streptavidin-conjugated QDs were first used for imaging of the tumor marker HER2/neu on the surface of human breast tumor SKBR-3 cells through biotin-conjugated antihuman secondary antibodies and humanized anti-HER2/neu antibodies. For all intended targets, specific labeling signals were brighter and considerably more photo-stable in comparison to the organic dye methods [53]. Multiplexed QDs diagnostics for the marker-molecules may be applicable for high antigen expression pattern contents analysis in the primary tumor tissue radically increasing the diagnostic value of the assays, elucidating differences in patterns and reflecting individual molecular signatures of events like microinvasions, etc. [54]. Since QDs were first introduced, their use in biologic experiments has become common, and important insights in cell biology have been made. For instance, QDs were used for the first time to document the dynamics of receptor transport at the cell membrane level. QDs have also been used to deliver and monitor siRNA in cells [55].
For the molecular genomic diagnosis, QDs conjugated oligonucleotide probes to visualize and map out the genetic material by FISH could identify the genes expressed even at very low levels [56]. In breast cancer, the QD based fluorescent probes were found to be more sensitive than traditional tags for FISH detections of HER2/neu oncogene, single-nucleotide polymorphism of cytochrome p450, and p53 tumor suppressor gene [57,58]. Recently, the HER had been under research focus as a likely dependable cancer marker, including breast cancer. QDs tagged with the EGF staining for the HER2 membrane receptor in cultured human cancer cells presented astonishing sensitive results [59]. QDs genomic analysis was also applicable in the in vitro studies of human metaphase chromosomes in transformed lymphocyte culture and breast cancer cell lines. In addition to tissue diagnostics, there are also emerging applications of QDs for in vitro detection assays and many of them are fluorescence resonance energy transfer based DNA hybridization applications [60]. For the targeted gene expression silencing therapy, the QDs conjugated silencing RNA (HER2-siRNA) may be tracked for delivery of the therapeutic siRNA and monitored for effectiveness of the siRNA-mediated down regulation of concerned receptor in the breast cancer cell lines [61].
Molecular imaging in combination with anatomical imaging, such as CT or positron emission tomography (PET)/CT, enables characterization of the molecular status of deep seated tumors. Their dependence on radioactivity-based methods has certain drawbacks, like short isotope half-lives, lack of multiplexing capability and low spatial resolution. Deep-tissue multiphoton microscopy, can image cells in three dimensions with high sensitivity and high spatial and temporal resolution. However, tissue penetrance of light is low, even for near infrared light, whilst conventional fluorophores are of insufficient brightness or stability for efficient visualization. QDs overcome these problems and have therefore been used extensively in live animal imaging, enabling detection of tumors at the deep sites. Large amounts of QDs can be transferred into live mammalian cells, either by nonspecific pinocytosis, microinjection or peptide-induced transport and up to two billion QDs have been delivered to the nucleus of a single cell without altering the cellular function or viability. Such labeled cells have been used to study embryogenesis [62], cancer metastases [63], stem cell therapy, and lymphocyte homing. They are a particularly powerful tool for embryogenesis and stem cell research, where multiplexing is extremely advantageous given the scarcity of tissue in such situations, whilst stem cells are rare and often require multiple markers for their correct identification. More important use of QDs is for lymph node mapping in cancer. QDs were used to perform simultaneous multicolor imaging of five different lymphatic basins as a tool for mapping lymphatic flow. Near infrared QDs were also used for sentinel node mapping in cancer surgery in animals. QDs were injected intradermally in the distal extremities and imaging methods were used to track their movement along the lymphatic channels, with identification of the sentinel lymph node. Furthermore, these experiments demonstrated high contrast between autofluorescence and emission signal, allowing minimal surgical incision for removal of the positive sentinel node [64]. Later, fluorescent tracking of solubilized NIR QDs injected subcutaneously in the anterior pawn mice demonstrated accumulation in the regional lymph nodes within 5 minutes of injection, and with a maximum concentration after 4 hours of injection, which then gradually falls down in the next 10 days, with resultant low-level uptake in other organs. Tracking, using fluorescent imaging, was compared with inductively coupled plasma-mass spectroscopy, demonstrating advantages of fluorescent imaging. These experiments were performed in mice and pigs, demonstrating their applicability for larger mammals, raising the possibility of intraoperative use in humans [65]. Such an approach would be particularly useful in breast cancer surgery, in which the sentinel node mapping is very common. QDs have also been used to image blood vessels in live mice, which demonstrated higher contrast and imaging depth than previously achieved with organic fluorophores. QDs have also been used to track cancer metastases, either by labeling antibodies reactive against cancer cells, or by direct labeling of cancer cells. Noh et al. [66] used QD-labeled dendritic cells to track their migration, following injection into mice foot pads, demonstrating that their final destination was popliteal or inguinal lymph nodes. This study demonstrated the ability of QD labeling to track immunotherapeutic cells, of importance for understanding the novel dendritic cell-based vaccination. These not only allow elegant imaging, but also enable real-time studies to be performed without frequent animal sacrifice, with advantages of improved experimental control and reduced cost and suffering, these factors could be vital for experimental feasibility. QDs with appropriate ligands as effecter molecules provide means for 'prolonged real-time visualizations' of the multistep signaling mechanisms in the living cell, and affording detailed movies of the processes involved. Cancer cell targeting by drug/Herceptin and its tracking by single QD conjugation with monoclonal anti-HER2 Ab was also a success in mice [67]. Various stages, which were visualized included: circulation in blood vessel, extravasation from blood vessel, presence in extracellular region, binding to HER2 on the cell membrane, moving from cell membrane to perinuclear region and finally presence in perinuclear region real-time imaging, using QDs, has an inherent limitation of autofluorescence (background signal) from intrinsic fluorophores in the normal tissue, lowering the target-to-background ratio. Several new technologies have been developed to reduce the autofluorescence. For instance, upconverting nanoparticles (UCNPs) are unique nanocrystals that emit light at shorter wavelengths than the excitation light in the NIR. This is achieved by converting energy of two photons serially absorbed by the UCNP. Conventional fluorophores, including QDs, typically emit light at longer wavelengths than the excitation light. Although, the shorter wavelength excitation light also excites autofluorescence, but in UCNPs, the longer wavelength excitation light (in the NIR) avoids exciting autofluorescence, and therefore, the shorter wavelength emission light is easily detectable. Optical lymphangiography in mice was successfully performed; superficial neck lymph nodes of the mice were depicted with no background signal in the visible range or NIR range. Furthermore, with serial injections of both the longer wavelength NIR and visible light, UCNPs lymphatic imaging was achieved in two colors simultaneously without a background signal.
In another approach to reduce the autofluorescence, QDs have been conjugated with luciferase, enabling self-illumination based on the principle of bioluminescence resonance energy transfer (BRET). The fluorescence emitted by these QDs can be illuminated by the bioluminescence produced by the reaction between luciferase and coelenterazine around the QD surface. Therefore, self-illuminating QDs can emit bright fluorescence with no requirement for external excitation, which exhibits extremely high sensitivity for use in vivo imaging. Such a bioluminescent approach enables QDs to be visualized at the deep sites where incident excitation light is low. Experiments on mice were performed to look for the feasibility of self-illuminating QDs. In all experimental mice, the SLN in each lymphatic basin were clearly visualized with no background signals. Sufficient signal for imaging was present for at least 30 minutes after coelenterazine injection. Moreover, by changing the QDs within the BRET-QD conjugate, it is possible to alter the emission wavelength, enabling multicolor in vivo lymphatic imaging with BRET-QDs [68]. QDs have a short half-life in circulation, due to hepatic uptake and efforts are going on to increase half-life by attachment of passivating molecules, such as polyethylene glycol, though this introduces further toxicity issues. Gao et al. [69] generated mercaptopropionic acid coated InAs/InP/ZnSe QDs with enhanced permeability and retention in vivo. They have an emission wavelength of approximately 800 nm and a very small hydrodynamic diameter (less than 10 nm) enhancing cellular uptake. They were highly accumulated in tumor xenografts in living mice, whilst additional coating with human serum albumin reduced localization in macrophages, and therefore, in the reticuloendothelial system, thus increasing their relative accumulation in tumors. There is an increasing body of work detailing the generation of multimodal QDs capable of both in vivo tumor cell tracking and of drug delivery. Weng et al. [70] conjugated liposomes to QDs together with anti-HER2 antibody, using the liposomes for doxorubicin loading, showing efficient anticancer activity in HER2 overexpressing breast cancer cells, and enabling tumor cell imaging. Recently, Harbeck and Thomssen [71] reported that node-negative breast cancer does not automatically suggest a good prognosis, or preclusion of chemotherapy benefits. Tumor-grade, Adjuvant! online algorithm, urokinase-type plasminogen activator could not be standard or dependable parameters to decide about the inclusion/exclusion of chemotherapy, hence, additional biomarkers are needed to help identify those patients who will actually benefit from chemotherapy. The dilemma that clinicians now face is about 30% of those node-negative patients who need chemotherapy because of their real chance of cancer recurrence, but there are no tools currently available to identify this subset of patients. In such cases that are declared as node negative by histopathology and other traditional IHC staining methods, QDs based applications may have a role to screen the SLNs, and have potential of elaborating any micrometastases >0.2 mm size as an authentic evidence to the determine need of adjuvant therapy for prevention of recurrence, and better cure. With the results obtained by QDs approach, a multiparametric tool for tumor cells detection is likely to be developed and implemented for early cancer and detection of micrometastases for monitored targeted cancer therapy.
The major limitation shared by all micrometastases detection techniques is a relatively poor tumor cell yield and the low detection limit of labeled cells. Thus, quantitative micrometastases diagnosis remains difficult to be resolved with present traditional means. A more effective approach, based on nanoparticles for biomarkers detection, would increase the diagnostic accuracy. As mentioned earlier, advantages of semiconductor fluorescent QDs over organic dyes include large absorption coefficients across a wide spectral range, size and composition tunable fluorescence emission, multiplexing characteristics (allowing simultaneous detection of several biomarkers), and very high levels of brightness and photostability, etc. Owing to these properties, QDs allow detection at the single particle level, making it possible to carry out an analysis of low-abundant biomolecules and rare cells, such as that of micrometastases. Indeed, a single nanoparticle is large enough for conjugation with multiple ligands, leading to enhanced binding affinity and exquisite specificity through the multivalency effect. These features are especially important in the detection of micrometastases cancer biomarkers, which are expressed in low concentration by few cells only. The possibility of intravital labeling of tumors with QDs was first demonstrated on mouse models. It was demonstrated that after intravenous administration, QDs conjugated to peptides specific to various type of tumors and their vessels are selectively accumulated in the tumor vasculature. This progress in the in vivo application of QDs has stimulated a large number of studies devoted to the intravital imaging of human model tumors in animals using QDs targeted at different tumor markers. Full-size antibodies and their fragments, specific peptides, and natural ligands were used as targeting ligands with equal success. The use of targeted QDs as fluorophores, in combination with modern optical imaging methods, allows to perform imaging of not only solid tumors, but also metastases in other organs, bone tissue and to reveal micrometastases at the early stages of the disease [47]. Direct covalent binding and streptavidin-biotin linkage may be used to conjugate the QDs with a panel of mAbs raised against various cancer markers present on the micrometastatic cells and may potentially be used for in vivo and in vitro serum and tissue diagnosis of the micrometastases [23]. Recently, micrometastases from lung cancer have been successfully detected with the help of magnetic nanoparticles (MNP) and QDs. Pan-cytokeratin (pan-ck) antibody was coupled to the surface of the MNPs, lunx (a novel human lung specific gene), and surfactant protein-A (SP-A) Abs were attached to QDs. MNPs were initially used for cell enrichment, and QDs were later utilized for micrometastatic cell detection [72]. This successful research of micrometastases detection in lung cancer by QDs has provided a solid scientific evidence, raising the hope that soon in near future, QDs can be similarly used to detect micrometestases in breast cancer. Therefore, the morbidity causing ALND and highly toxic adjuvant chemotherapy would be given to those patients only who are positive for micrometastases. An early detection and adequate removal of the micrometastatic cells will lead to prevention of recurrence later.
QDs toxicity depends on multiple factors derived from both the inherent physicochemical properties of QDs and environmental conditions. QDs size, charge, concentration, outer coating bioactivity (capping material and functional groups), and oxidative, photolytic, and mechanical stability are factors that, collectively and individually, determine the QDs toxicity [73].
Breast cancer cure aims at complete elimination of the malignant cells from the breast and any other source(s) of metastatic deposit (like SLN, any other tissue/organ, etc.). The occult micrometastatic deposits remain the untreated source of potential subsequent relapses. The detection and characterization of these micrometastases in the sentinel node with the help of QDs should lead to a better understanding of fundamental mechanisms of metastatic spread in cancer patients and will eventually contribute to the development of more effective strategies to eliminate the micrometastatic cells. With QDs approach multi-parametric tool for tumor cells detection is likely to be developed for an early cancer diagnosis, mapping SLN, detection of micrometastases and monitored targeted cancer therapy. Since their first use for biological imaging in 2001, QDs have been used in a wide variety of in vitro and in vivo applications. There have been recent efforts to minimize their potential toxicity by novel formulation, and production of "small" QDs to facilitate molecular tracking. Sophisticated imaging systems are required for analysis of multiplexed images and the relative lack of such systems has hindered their more widespread use in vitro imaging, whilst the range of in vivo applications continues to grow almost exponentially, and solution of their potential toxicity will hopefully enable their clinical applications soon. Overall, whilst QDs have shown great promise in the scientific literature, this has not yet been translated into clinical usage, though the efforts being made to reduce toxicity, improve imaging systems, and standardize quantitation are expected to increase their clinical and translational use.
ACKNOWLEDGEMENTS
I am highly thankful to Prof. Mohammed Naim for his untiring and unending support towards me.
References
1. Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006; 98:599–609. PMID: 16670385.

2. Fantozzi A, Christofori G. Mouse models of breast cancer metastasis. Breast Cancer Res. 2006; 8:212. PMID: 16887003.

3. Meric F, Mirza NQ, Vlastos G, Buchholz TA, Kuerer HM, Babiera GV, et al. Positive surgical margins and ipsilateral breast tumor recurrence predict disease-specific survival after breast-conserving therapy. Cancer. 2003; 97:926–933. PMID: 12569592.

4. Sienel W, Stremmel C, Kirschbaum A, Hinterberger L, Stoelben E, Hasse J, et al. Frequency of local recurrence following segmentectomy of stage IA non-small cell lung cancer is influenced by segment localisation and width of resection margins: implications for patient selection for segmentectomy. Eur J Cardiothorac Surg. 2007; 31:522–527. PMID: 17229574.
5. Karni T, Pappo I, Sandbank J, Lavon O, Kent V, Spector R, et al. A device for real-time, intraoperative margin assessment in breast-conservation surgery. Am J Surg. 2007; 194:467–473. PMID: 17826057.

6. Neoptolemos JP, Stocken DD, Dunn JA, Almond J, Beger HG, Pederzoli P, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001; 234:758–768. PMID: 11729382.

7. Pantel K, Otte M. Occult micrometastasis: enrichment, identification and characterization of single disseminated tumour cells. Semin Cancer Biol. 2001; 11:327–337. PMID: 11562175.

8. Pantel K, Alix-Panabières C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009; 6:339–351. PMID: 19399023.

9. Braun S, Kentenich C, Janni W, Hepp F, de Waal J, Willgeroth F, et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol. 2000; 18:80–86. PMID: 10623696.
10. Kruger WH, Kröger N, Tögel F, Renges H, Badbaran A, Hornung R, et al. Disseminated breast cancer cells prior to and after high-dose therapy. J Hematother Stem Cell Res. 2001; 10:681–689. PMID: 11672515.
11. Sobin LH, Hermanek P, Hutter RV. TNM classification of malignant tumors. A comparison between the new (1987) and the old editions. Cancer. 1988; 61:2310–2314. PMID: 3284634.

12. Mittendorf EA, Hunt KK. Significance and management of micrometastases in patients with breast cancer. Expert Rev Anticancer Ther. 2007; 7:1451–1461. PMID: 17944569.

13. Schwartz GF, Giuliano AE, Veronesi U. Consensus Conference Committee. Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast, April 19-22, 2001, Philadelphia, Pennsylvania. Cancer. 2002; 94:2542–2551. PMID: 12173319.

14. Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005; 23:7703–7720. PMID: 16157938.

15. Kosaka N, McCann TE, Mitsunaga M, Choyke PL, Kobayashi H. Real-time optical imaging using quantum dot and related nanocrystals. Nanomedicine (Lond). 2010; 5:765–776. PMID: 20662647.

16. Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983; 52:1551–1557. PMID: 6352003.

17. Kahn HJ, Hanna WM, Chapman JA, Trudeau ME, Lickley HL, Mobbs BG, et al. Biological significance of occult micrometastases in histologically negative axillary lymph nodes in breast cancer patients using the recent American Joint Committee on Cancer breast cancer staging system. Breast J. 2006; 12:294–301. PMID: 16848838.

18. Gusterson BA, Ott R, Anderson TJ, Galea MH, Elston CW, Blamey RW. Occult axillary lymph-node micrometastases in breast cancer. Lancet. 1990; 336:434–435. PMID: 1974958.

19. Neville AM. Breast cancer micrometastases in lymph nodes and bone marrow are prognostically important. Ann Oncol. 1991; 2:13–14. PMID: 2009231.

20. Gebauer G, Fehm T, Merkle E, Beck EP, Lang N, Jäger W. Epithelial cells in bone marrow of breast cancer patients at time of primary surgery: clinical outcome during long-term follow-up. J Clin Oncol. 2001; 19:3669–3674. PMID: 11504748.

21. Landys K, Persson S, Kovarík J, Hultborn R, Holmberg E. Prognostic value of bone marrow biopsy in operable breast cancer patients at the time of initial diagnosis: results of a 20-year median follow-up. Breast Cancer Res Treat. 1998; 49:27–33. PMID: 9694608.

22. Gerber B, Krause A, Müller H, Richter D, Reimer T, Makovitzky J, et al. Simultaneous immunohistochemical detection of tumor cells in lymph nodes and bone marrow aspirates in breast cancer and its correlation with other prognostic factors. J Clin Oncol. 2001; 19:960–971. PMID: 11181658.
23. Mahmoud W, Sukhanova A, Oleinikov V, Rakovich YP, Donegan JF, Pluot M, et al. Emerging applications of fluorescent nanocrystals quantum dots for micrometastases detection. Proteomics. 2010; 10:700–716. PMID: 19953553.

24. Schwartz GF, Giuliano AE, Veronesi U. Consensus Conference Committee. Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast April 19 to 22, 2001, Philadelphia, Pennsylvania. Hum Pathol. 2002; 33:579–589. PMID: 12152156.

25. Pathology reporting of breast disease. 2005. Accessed June 13th, 2012. NHS Cancer Screening Programmes, The Royal College of Pathologists;http://www.cancerscreening.nhs.uk/breastscreen/publications/nhsbsp58-low-resolution.pdf.
26. Wells CA. Perry N, Broeders M, Wolf C, Tornberg S, Holland R, Karsa LV, editors. Open biopsy and resection specimens. European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis. 2006. 4th ed. Luxembourg: Office for Official Publications of the European Communities;p. 257–266.
27. Kuehn T, Bembenek A, Decker T, Munz DL, Sautter-Bihl ML, Untch M, et al. A concept for the clinical implementation of sentinel lymph node biopsy in patients with breast carcinoma with special regard to quality assurance. Cancer. 2005; 103:451–461. PMID: 15611971.

28. Meyer JS. Sentinel lymph node biopsy: strategies for pathologic examination of the specimen. J Surg Oncol. 1998; 69:212–218. PMID: 9881937.

29. Treseler P. Pathologic examination of the sentinel lymph node: what is the best method? Breast J. 2006; 12(5 Suppl 2):S143–S151. PMID: 16958994.

30. Millis RR, Springall R, Lee AH, Ryder K, Rytina ER, Fentiman IS. Occult axillary lymph node metastases are of no prognostic significance in breast cancer. Br J Cancer. 2002; 86:396–401. PMID: 11875706.

31. Willipinski-Stapelfeldt B, Riethdorf S, Assmann V, Woelfle U, Rau T, Sauter G, et al. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res. 2005; 11:8006–8014. PMID: 16299229.

32. Borgen E, Beiske K, Trachsel S, Nesland JM, Kvalheim G, Herstad TK, et al. Immunocytochemical detection of isolated epithelial cells in bone marrow: non-specific staining and contribution by plasma cells directly reactive to alkaline phosphatase. J Pathol. 1998; 185:427–434. PMID: 9828843.

33. Braun S, Pantel K. Micrometastatic bone marrow involvement: detection and prognostic significance. Med Oncol. 1999; 16:154–165. PMID: 10523795.

34. Noguchi S, Aihara T, Motomura K, Inaji H, Imaoka S, Koyama H. Detection of breast cancer micrometastases in axillary lymph nodes by means of reverse transcriptase-polymerase chain reaction. Comparison between MUC1 mRNA and keratin 19 mRNA amplification. Am J Pathol. 1996; 148:649–656. PMID: 8579127.
35. Masuda N, Tamaki Y, Sakita I, Ooka M, Ohnishi T, Kadota M, et al. Clinical significance of micrometastases in axillary lymph nodes assessed by reverse transcription-polymerase chain reaction in breast cancer patients. Clin Cancer Res. 2000; 6:4176–4185. PMID: 11106229.
36. Gillanders WE, Mikhitarian K, Hebert R, Mauldin PD, Palesch Y, Walters C, et al. Molecular detection of micrometastatic breast cancer in histopathology-negative axillary lymph nodes correlates with traditional predictors of prognosis: an interim analysis of a prospective multi-institutional cohort study. Ann Surg. 2004; 239:828–837. PMID: 15166962.
37. Viale G, Dell'Orto P, Biasi MO, Stufano V, De Brito Lima LN, Paganelli G, et al. Comparative evaluation of an extensive histopathologic examination and a real-time reverse-transcription-polymerase chain reaction assay for mammaglobin and cytokeratin 19 on axillary sentinel lymph nodes of breast carcinoma patients. Ann Surg. 2008; 247:136–142. PMID: 18156933.

38. Visser M, Jiwa M, Horstman A, Brink AA, Pol RP, van Diest P, et al. Intra-operative rapid diagnostic method based on CK19 mRNA expression for the detection of lymph node metastases in breast cancer. Int J Cancer. 2008; 122:2562–2567. PMID: 18324628.

39. Ring A, Smith IE, Dowsett M. Circulating tumour cells in breast cancer. Lancet Oncol. 2004; 5:79–88. PMID: 14761811.

40. Balducci E, Azzarello G, Valori L, Toffolatti L, Bolgan L, Valenti MT, et al. A new nested primer pair improves the specificity of CK-19 mRNA detection by RT-PCR in occult breast cancer cells. Int J Biol Markers. 2005; 20:28–33. PMID: 15832770.

41. von Burstin J, Eser S, Seidler B, Meining A, Bajbouj M, Mages J, et al. Highly sensitive detection of early-stage pancreatic cancer by multimodal near-infrared molecular imaging in living mice. Int J Cancer. 2008; 123:2138–2147. PMID: 18709639.

42. Rhyner MN, Smith AM, Gao X, Mao H, Yang L, Nie S. Quantum dots and multifunctional nanoparticles: new contrast agents for tumor imaging. Nanomedicine (Lond). 2006; 1:209–217. PMID: 17716110.

43. Smith AM, Duan H, Mohs AM, Nie S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev. 2008; 60:1226–1240. PMID: 18495291.

44. Peng CW, Li Y. Application of quantum dots-based biotechnology in cancer diagnosis: current status and future perspectives. J Nanomater. 2010; 2010:Article ID 676839.

45. Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007; 9:257–288. PMID: 17439359.

46. Fountaine TJ, Wincovitch SM, Geho DH, Garfield SH, Pittaluga S. Multispectral imaging of clinically relevant cellular targets in tonsil and lymphoid tissue using semiconductor quantum dots. Mod Pathol. 2006; 19:1181–1191. PMID: 16778828.

47. Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003; 7:626–634. PMID: 14580568.

48. Zdobnova TA, Lebedenko EN, Deyev Scapital Em C. Quantum dots for molecular diagnostics of tumors. Acta Naturae. 2011; 3:29–47. PMID: 22649672.

49. Yezhelyev MV, Al-Hajj A, Morris C, Marcus AI, Liu T, Lewis M, et al. In situ molecular profiling of breast cancer biomarkers with multicolor quantum dots. Adv Mater. 2007; 19:3146–3151.

50. Ko MH, Kim S, Kang WJ, Lee JH, Kang H, Moon SH, et al. In vitro derby imaging of cancer biomarkers using quantum dots. Small. 2009; 5:1207–1212. PMID: 19235198.

51. Chen C, Peng J, Xia HS, Yang GF, Wu QS, Chen LD, et al. Quantum dots-based immunofluorescence technology for the quantitative determination of HER2 expression in breast cancer. Biomaterials. 2009; 30:2912–2918. PMID: 19251316.

52. Lidke DS, Nagy P, Heintzmann R, Arndt-Jovin DJ, Post JN, Grecco HE, et al. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nat Biotechnol. 2004; 22:198–203. PMID: 14704683.

53. Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003; 21:41–46. PMID: 12459735.

54. Jan E, Byrne SJ, Cuddihy M, Davies AM, Volkov Y, Gun'ko YK, et al. High-content screening as a universal tool for fingerprinting of cytotoxicity of nanoparticles. ACS Nano. 2008; 2:928–938. PMID: 19206490.

55. Yezhelyev MV, Qi L, O'Regan RM, Nie S, Gao X. Proton-sponge coated quantum dots for siRNA delivery and intracellular imaging. J Am Chem Soc. 2008; 130:9006–9012. PMID: 18570415.

56. Shi C, Zhu Y, Cerwinka WH, Zhau HE, Marshall FF, Simons JW, et al. Quantum dots: emerging applications in urologic oncology. Urol Oncol. 2008; 26:86–92. PMID: 18190836.

57. Xiao Y, Telford WG, Ball JC, Locascio LE, Barker PE. Semiconductor nanocrystal conjugates, FISH and pH. Nat Methods. 2005; 2:723. PMID: 16179915.

58. Xu H, Sha MY, Wong EY, Uphoff J, Xu Y, Treadway JA, et al. Multiplexed SNP genotyping using the Qbead system: a quantum dot-encoded microsphere-based assay. Nucleic Acids Res. 2003; 31:e43. PMID: 12682378.
59. Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006; 7:131–142. PMID: 16493418.

60. Zhang CY, Johnson LW. Quantifying RNA-peptide interaction by single-quantum dot-based nanosensor: an approach for drug screening. Anal Chem. 2007; 79:7775–7781. PMID: 17877365.

61. Tan WB, Jiang S, Zhang Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials. 2007; 28:1565–1571. PMID: 17161865.

62. Murasawa S, Kawamoto A, Horii M, Nakamori S, Asahara T. Niche-dependent translineage commitment of endothelial progenitor cells, not cell fusion in general, into myocardial lineage cells. Arterioscler Thromb Vasc Biol. 2005; 25:1388–1394. PMID: 15860746.

63. Voura EB, Jaiswal JK, Mattoussi H, Simon SM. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat Med. 2004; 10:993–998. PMID: 15334072.

64. Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004; 22:93–97. PMID: 14661026.

65. Soltesz EG, Kim S, Laurence RG, DeGrand AM, Parungo CP, Dor DM, et al. Intraoperative sentinel lymph node mapping of the lung using near-infrared fluorescent quantum dots. Ann Thorac Surg. 2005; 79:269–277. PMID: 15620956.

66. Noh YW, Lim YT, Chung BH. Noninvasive imaging of dendritic cell migration into lymph nodes using near-infrared fluorescent semiconductor nanocrystals. FASEB J. 2008; 22:3908–3918. PMID: 18682573.

67. Tada H, Higuchi H, Wanatabe TM, Ohuchi N. In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Res. 2007; 67:1138–1144. PMID: 17283148.

68. So MK, Xu C, Loening AM, Gambhir SS, Rao J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat Biotechnol. 2006; 24:339–343. PMID: 16501578.

69. Gao J, Chen K, Xie R, Xie J, Lee S, Cheng Z, et al. Ultrasmall near-infrared non-cadmium quantum dots for in vivo tumor imaging. Small. 2010; 6:256–261. PMID: 19911392.

70. Weng KC, Noble CO, Papahadjopoulos-Sternberg B, Chen FF, Drummond DC, Kirpotin DB, et al. Targeted tumor cell internalization and imaging of multifunctional quantum dot-conjugated immunoliposomes in vitro and in vivo. Nano Lett. 2008; 8:2851–2857. PMID: 18712930.

71. Harbeck N, Thomssen C. A new look at node-negative breast cancer. Oncologist. 2011; 16(Suppl 1):51–60. PMID: 21278441.

72. Wang Y, Zhang Y, Du Z, Wu M, Zhang G. Detection of micrometastases in lung cancer with magnetic nanoparticles and quantum dots. Int J Nanomedicine. 2012; 7:2315–2324. PMID: 22661888.
73. Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006; 114:165–172. PMID: 16451849.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download