Abstract
On a pathological specimen of breast cancer cells, retraction artifact during histological processing mimics true lymphovascular invasion (LVI). The accurate determination of the presence or absence of LVI is a factor in determining risk of having a positive sentinel node, or having additional positive axillary nodes after a positive sentinel node biopsy in women with early-stage breast cancer. The determination of nodal risk influences the decision of the treating physicians as to whether a sentinel node biopsy or completion axillary dissection is necessary. On slide preparation, ideal factors favoring true LVI include: a definite endothelial lining, with endothelial nuclei that seem to protrude into the lymphatic space; invasion in one lymphatic vessel (LV) lumen with nearby cancer glands that have minimal or no retraction; a tumor embolus in a LV clear lumen with outside nearby tumor bulk; a tumor embolus that is different in shape than its surrounding clear LV space; and a positive stain for fibrin, CD31, or CD34 on tumor embolus periphery.
Lymphovascular invasion (LVI) refers to the presence of tumor cells within vascular spaces (i.e., lymphatics or small capillaries) surrounding tumors. The finding of LVI is associated with a higher risk of lymph node metastases and is a poor prognostic factor in women without lymph node metastases [1]. For example, among patients with T1N0M0 breast cancer, there is an increased frequency of recurrence and death due to breast carcinoma with LVI [2]. The proper determination of the presence or absence of LVI may have clinical significance in medical decision making as well. The accurate determination of the presence or absence of LVI is a factor in determining risk of having a positive sentinel node [3], or having additional positive axillary nodes after a positive sentinel node biopsy [4] in women with early-stage breast cancer. The determination of nodal risk influences the decision of the treating physicians as to whether a sentinel node biopsy or completion axillary dissection is necessary.
Recognition of lymphatic tumor emboli on microscopic section is dependent on the pathologist. There is a potential for significant inter-observer variation in the diagnosis of LVI amongst pathologists. For instance, Gilchrist et al. [5] noted that when three surgical pathologists were told to assess for LVI in a pT1-2 N0 M0 histological mastectomy case, all three concurred in only 12 of 35 cases. One source of inter-observer variation is retraction artifact during histological processing that mimics true LVI. Though retraction artifact was previously considered an insignificant finding that interfered with the ability to make an appropriate diagnosis [6], more recent studies have suggested it is not a random phenomenon from tissue fixation. Instead, it may be a result of tumor-stromal interactions suggesting a poor prognosis [7,8]. The purposes of this article are to provide an illustrative description comparing true LVI to retraction artifact and provide insight of quantifying the significance of retraction artifact in future studies.
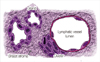
In breast stroma, cancer cells form in clusters near lymphatic vessels and as glands (Figure 1). The term "lymphatic vessel" refers to a lymph vessel or capillary; these vessels are endothelial-lined channels, devoid of red blood cells, and lacking a smooth muscle cell wall [2]. In true LVI, cancer cells break through the basement membrane. The lumenal invasion is a transient event; thus, it is rarely captured on histologic specimen. A tumor embolus forms in the lumen. On slide preparation, ideal factors favoring true LVI include: 1) a definite endothelial lining, with endothelial nuclei that seem to protrude into the lymphatic space; 2) invasion in one lymphatic vessel (LV) lumen with nearby cancer glands that have minimal or no retraction; 3) a tumor embolus in a LV clear lumen with outside nearby tumor bulk; 4) a tumor embolus that is different in shape than its surrounding clear LV space; and 5) a positive stain for fibrin, CD31, or CD34 on tumor embolus periphery [9,10].
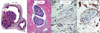
Figure 2A is an illustration of the ideal characteristics favoring true LVI. Figure 2B shows pathological specimens of true LVI stained with hematoxylin and eosin (H&E, at ×40 magnification), and with CD31 or CD34 (both at ×20 magnification, with detail emphasizing the lymphatic space involved). The involved lymphatic space is often in immediate proximity of an artery and a nerve trunk, histologic features very helpful in identifying LVI with certainty, but depending on the plane of section not all three elements are always present on the slide. For example Figure 2 illustrates LVI and an adjacent artery, but no nerve trunk is present in the plane of section. Figure 3A is an illustration of the ideal characteristics that favor retraction artifact. Figure 3B shows three cases of pathological specimens of retraction artifact stained with H&E.
A variable degree of retraction artifact may be seen in 16% to 60% of invasive breast carcinomas [8,11]. Acs et al. [11] found that extensive retraction artifact stained with D2-40, especially in pT1 and pT2 breast carcinomas, correlates with tumor size, histologic type, histologic grade, presence of lymphatic spread, and poor outcome. Irie et al. [8] observed that retraction artifact was seen in 84% of cases of infiltrating ductal carcinoma and similarly suggested that retraction might represent true prelymphatic space involvement. Though the findings are interesting, they are limited. For example, in the Acs et al. [11] study, D2-40 may not have been an ideal marker for finding LVI, as D2-40 staining of myoepithelial cells and myofibroblasts at the edge of retraction spaces of ductal carcinoma in situ may be misinterpreted as tumor LVI [12], and the direct destruction of the endothelial cell layer by enzymatic digestion of matrix proteins by the neoplastic cells can result in lack of expression of D2-40 [8]. In the Irie et al. [8] study, retraction of >25% of the specimen was seen in 168 of 199 infiltrating ductal carcinoma specimens, but only 1 of 188 ductal carcinoma in situ specimens.
Thus, it is unclear how retraction artifact should be considered in cancer staging (e.g., T or N stage). Multiple stains (e.g., with type IV collagen, cytokeratin, and smooth muscle actin) have been recommended in LVI diagnosis [13,14]. A combination scoring system will likely be necessary in future studies of both LVI and retraction artifact to more clearly prognosticate the incidence of tumor metastasis. To further the assess the significance of retraction artifact, we recommend: 1) quantifying the instances of retraction artifact in one specimen; 2) noting tumor histology; 3) using more than one cellular marker in preparation; and 4) grading the size of the largest glands undergoing LVI, as these may be associated with a stronger force of tumor-stromal interaction.
Figures and Tables
References
1. Lester SC. Kumar VK, Abbas AK, Fausto N, editors. The breast. Robbins and Cotran's Pathological Basis of Disease. 2005. 7th ed. Philadelphia: Elsevier, Saunders;1148.

2. Rosen PP. Tumor emboli in intramammary lymphatics in breast carcinoma: pathologic criteria for diagnosis and clinical significance. Pathol Annu. 1983. 18 Pt 2:215–232.
3. Bevilacqua JL, Kattan MW, Fey JV, Cody HS 3rd, Borgen PI, Van Zee KJ. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol. 2007. 25:3670–3679.

4. Van Zee KJ, Manasseh DM, Bevilacqua JL, Boolbol SK, Fev JV, Tan LK, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003. 10:1140–1151.

5. Gilchrist KW, Gould VE, Hirschl S, Imbriqlia JE, Patchefsky AS, Penner DW, et al. Interobserver variation in the identification of breast carcinoma in intramammary lymphatics. Hum Pathol. 1982. 13:170–172.

6. Yeh I, Brooks JSJ, Pietra GG. Atlas of Microscopic Artifacts and Foreign Materials. 1997. 1st ed. Baltimore: Williams & Wilkins;144.
7. Acs G, Paragh G, Chuang ST, Laronga C, Zhang PJ. The presence of micropapillary features and retraction artifact in core needle biopsy material predicts lymph node metastasis in breast carcinoma. Am J Surg Pathol. 2009. 33:202–210.

8. Irie J, Manucha V, Ioffe OB, Silverberg SG. Artefact as the pathologist's friend: peritumoral retraction in in situ and infiltrating duct carcinoma of the breast. Int J Surg Pathol. 2007. 15:53–59.

9. Lee AK, DeLellis RA, Wolfe HJ. Intramammary lymphatic invasion in breast carcinomas. Evaluation using ABH isoantigens as endothelial markers. Am J Surg Pathol. 1986. 10:589–594.

10. Saigo PE, Rosen PP. The application of immunohistochemical stains to identify endothelial-lined channels in mammary carcinoma. Cancer. 1987. 59:51–54.

11. Acs G, Dumoff KL, Solin LJ, Pasha T, Xu X, Zhang PJ. Extensive retraction artifact correlates with lymphatic invasion and nodal metastasis and predicts poor outcome in early stage breast carcinoma. Am J Surg Pathol. 2007. 31:129–140.

12. Ren S, Abuel-Haija M, Khurana JS, Zhang X. D2-40: an additional marker for myoepithelial cells of breast and the precaution in interpreting tumor lymphovascular invasion. Int J Clin Exp Pathol. 2011. 4:175–182.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download