Abstract
Bone is the most common metastatic organ in patients with breast cancer. The most significant clinical symptom of bone metastasis is pain which reduces quality of life in cancer patients. We report a case of chest wall reconstruction after partial sternal resection for solitary sternal metastasis and modified radical mastectomy in a patient with locally advanced breast cancer. The sternal defect was reconstructed with a 2 mm thick Gore-Tex patch. Postoperative pain was acceptable and the patient was discharged without any complications. The patient received the endocrine and bisphosphonate therapy combined with chemotherapy and radiotherapy. No recurrence or complications were observed during a follow-up period of 36 months. We describe our good surgical management results of sternal metastatic lesion in a patient with locally advanced breast cancer. We suggest that simultaneous sternectomy is a safe and curative surgical method for a solitary sternal metastasis when no evidence of systemic spread is noted.
Breast cancer most commonly metastasizes to bone, followed by the lung, brain, and liver. Once bone metastasis has occurred, a complete cure is very difficult and assessment of the therapeutic response is limited [1]. Thus, the treatment focus for bone metastasis has been on palliative care rather than a cure. However, considering the increasing number of younger patients with breast cancer, a more aggressive treatment approach may be appropriate for patients with metastatic disease limited to a solitary lesion.
An isolated sternal metastasis occurs at an incidence rate of 1.9% to 2.4%. This type of metastasis has been considered regional lymphatic tumor spread rather than hematogenous dissemination because of the unique tendency to remain solitary for a longer time [2]. If no evidence of other distant metastasis except the sternum is present in a patient with metastatic breast cancer, the sternum may be suitable for resection with potentially good results. Improved diagnostic, staging, and surgical techniques may allow curative surgery in carefully selected patients who have a good performance status and a strong desire for radical surgical treatment with low morbidity and mortality [3].
We could not find any report of patients with a solitary sternal metastasis from breast cancer who underwent breast and sternum surgical treatment simultaneously. We describe our experience of a simultaneous operation, chest wall reconstruction after sternectomy and modified radical mastectomy, in a patient with locally advanced breast cancer and a solitary sternal metastasis.
A 28-year-old woman was admitted for left breast cancer with nipple retraction, palpable axillary lymph nodes, and dull pain in the upper-mid chest. Mammography revealed ill-defined hyperdense mass involving left whole breast with nipple retraction and diffuse skin and trabecular thickening with enlarged lymph nodes in left axilla. Breast ultrasonography showed an ill-defined irregular mass involving the entire left breast with diffuse skin thickening and nipple retraction and multiple malignant looking axillary lymph nodes were apparent. An image-guided core biopsy showed invasive ductal carcinoma and image-guided fine needle aspiration revealed metastatic ductal carcinoma in the axillary lymph node. The tumor was positive for the estrogen receptor and progesterone receptor but negative for human epidermal growth factor receptor type 2 (HER2). Initial breast magnetic resonance imaging (MRI) showed an enhanced nodule in the sternal body, caused by metastasis (Figure 1A). But, initial 18F-fluoro-2-D-glucose positron emission tomography with computed tomography (18FDG PET/CT) and bone scintigraphy showed negative findings in the sternum, and only the left highest mediastinal area and left supraclavicular lymph nodes metastasis appeared on the 18FDG-PET/CT (Figure 1B, C). The patient underwent palliative chemotherapy consisting of 50 mg/m2 body surface area (BSA) doxorubicin and 75 mg/m2 BSA docetaxel, every 3 weeks. Efficacy was assessed as a partial response on breast MRI after four cycles. The breast MRI showed an interval decrease in the entire left breast mass, left multiple metastatic axillary lymph nodes and metastatic nodule at the sternal body (Figure 2). She elected to have surgery after 4 months from her initial presentation. A left modified radical mastectomy with level III axillary lymph nodes dissection and partial sternectomy with chest wall reconstruction were performed (Figure 3). After longitudinal division of the pectoralis major muscle, periosteal elevation around the metastatic lesion including the third and fourth ribs was performed. A frozen section of the sternal mass was revealed metastatic ductal carcinoma. A subtotal sternectomy including the lower half of the sternal body was performed with an osteotome and oscillating saw. The resection field was over the left third and fourth costal cartilage margins of the intercostal muscles and the enlarged parasternal lymph nodes but excluded the unaffected lateral part of the pectoralis major muscles. After we identified that the frozen section result for resection margin was clear, we immediately repaired the large anterior chest-wall defect with a 2 mm thick polytetrafluoroethylene Gore-Tex soft tissue patch (W. L. Gore & Associates, Flagstaff, USA), which was sutured to the ribs, rectus abdominis muscles, and the remaining part of the sternum. The soft tissue was covered smoothly with layer by layer sutures of the pectoralis major muscles and subcutaneous tissue once the Gore-Tex patch was sutured in place. Microscopic examination of the breast and bone marrow sections revealed clusters of infiltrating ductal carcinoma (Figure 4A, B). She was extubated immediately after surgery with no postoperative complications. The patient did not have any problems with daily activities or chest movement. She was discharged from the hospital 10 days after the operation. Pathology revealed a 10.2×9.5×3.2 cm sized invasive ductal carcinoma with no involvement of the skin or nipple, but 12 axillary lymph nodes were involved along with the sternal metastasis. She received two additional cycles of chemotherapy followed by radiotherapy. We checked 18FDG-PET/CT and bone scintigraphy to confirm the surgical site and the sternum before and after radiotherapy (Figures 5, 6). The patient underwent the endocrine and bisphosphonate therapy and was well 36 months after the diagnosis. She exhibited no evidence of recurrent disease on the last imaging study, 18FDG-PET/CT, bone scintigraphy, or breast MRI (Figure 7). She is being followed up carefully every 4 weeks.
Bone metastasis occurs in up to 70% of patients with advanced breast cancer. Until now, the goals of metastatic bone treatment have remained pain relief, maintenance of function, and avoiding hypercalcemia, bone marrow invasion, and pathological fractures. More widespread use of sensitive imaging techniques such MRI and PET/CT has created the ability to detect bone metastasis at an earlier stage of asymptomatic disease. Provided that the primary breast cancer is controlled, there is a long disease-free period, and the patient has a good performance status, surgery is an important component in the multimodality approach to asymptomatic solitary bone metastasis. Sternectomy for isolated breast cancer metastasis remains a controversial issue. But, a single metastatic lesion in the sternum has a unique tendency to remain solitary for a longer time rather than to spread to other sites [4]. A solitary sternal metastasis could be regarded as a local problem rather than a systemic problem [2]. Thus, surgical resection of a single sternal metastatic lesion has curative intent. In our case, we considered two factors when deciding surgical treatment for the solitary sternal metastasis. One was the tumor factor. We considered the aggressive character of her tumor, because of cancer in young age and high tumor stage. The other was the patient factor. We considered her low quality of life, because of young age and chest pain. Our patient was treated with neoadjuvant chemotherapy and the breast became suitable for resection. She had good performance status and desperately wanted a complete cure. Veronesi et al. [5] reported that chest wall resection for locally recurrent or advanced breast cancer was a safe procedure with low morbidity and mortality that can provide good symptom palliation in patients with locally advanced breast cancer. An en bloc sternal resection for a primary or secondary tumor enabled the patient to obtain a better quality of life and long-term survival [6]. In the case of sternal neoplasms, a broad sternal resection followed by reconstruction with prosthetic materials is an effective and safe solution that considerably improves patient quality of life [7]. Various techniques for chest wall reconstruction after sternal resection have been described. It is probably better to use a rigid prosthesis to prevent paradoxical movement of the thorax when the lower part of the sternum is resected. However, soft mesh can be used when the upper part of the sternum is resected [8]. The first report on the use of a Gore-Tex soft-tissue patch without additional prosthetic material after subtotal sternectomy was in 1993 [9]. Gore-Tex has the advantage of being impermeable to air and liquids and provides excellent results in terms of stability, intrathoracic organ protection, and pulmonary expansion [10]. We performed a modified radical mastectomy and immediate chest wall reconstruction simultaneously using a Gore-Tex patch after partial sternectomy. The postoperative course was uneventful and additional treatment with chemotherapy and radiotherapy was completed. Until now, our patient is alive with a good quality of life without any symptoms or chest dysfunction at 36 months post-operatively. Long-term follow-up will be needed to verify whether this method prolongs survival. Nevertheless, we carefully suggest that surgical extirpation should be considered as a curative measure for patients with good performance status who have solitary sternal metastasis with no evidence of other spread. This multimodality treatment has resulted in an increased disease-free period and improved quality of life.
Figures and Tables
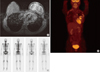 | Figure 1Initial breast imagings. (A) Breast magnetic resonance imaging shows diffuse enhanced left breast cancer involving whole breast with extensive axillary lymph nodes metastases and enhancing nodule in sternum body. (B) 18F-fluoro-2-D-glucose positron emission tomography with computed tomography shows multiple hypermetabolic lesions in left breast and lymph nodes in left axilla, left highest mediastinal area and left supraclavicular area and negative finding in sternum. (C) Bone scintigraphy shows no evidence of bone metastasis. |
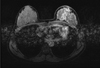 | Figure 2Breast magnetic resonance imaging followed after 4 cycle of palliative chemotherapy. It shows interval decrease of clumped heterogeneous non-mass-like enhancement involving left whole breast and in size of multiple metastatic lymph nodes at left axilla and metastatic nodule at sternum body. |
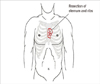 | Figure 3The illustration of subtotal sternectomy for metastatic lesion locating on the left side of sternal body at the level of 3rd chondrosternal junction area. |
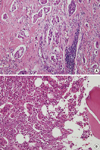 | Figure 4Microscopic findings. (A) Breast specimen revealed clusters of infiltrating ductal carcinoma (H&E stain, ×200). (B) Bone marrow specimen of sternum revealed clusters of infiltrating ductal carcinoma (H&E stain, ×200). |
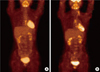 | Figure 5Postoperative imaging study of 18F-fluoro-2-D-glucose positron emission tomography with computed tomography. (A) Before radiotherapy, 2 months after surgery, it showed mild hypermetabolic activity at sternectomy site, which is caused by postoperative change. (B) After radiotherapy, 12 months after surgery, it showed decreased hypermetabolic activity at sternectomy site. |
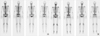 | Figure 6Postoperative imaging study of bone scintigraphy. (A) Before radiotherapy, 2 months after surgery, it showed increased uptake at sternectomy site, which is caused by postoperative change. (B) After radiotherapy, 12 months after surgery, there was no uptake change at sternectomy site. |
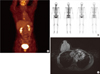 | Figure 7Recent breast imagings, 36 months after surgery. (A) 18F-fluoro-2-D-glucose positron emission tomography with computed tomography showed mild focal hypermetabolic lesion at sternum, which is probably postoperative change in sternum rather than recurrence. (B) Bone scintigraphy showed mild focal uptakes at sternum, which is probably postoperative change in sternum rather than recurrence. (C) Breast magnetic resonance imaging showed regional mild enhancement of operative site at sternum, which is probably postoperative change in sternum rather than recurrence. |
References
1. Kwai AH, Stomper PC, Kaplan WD. Clinical significance of isolated scintigraphic sternal lesions in patients with breast cancer. J Nucl Med. 1988. 29:324–328.
2. Noguchi S, Miyauchi K, Nishizawa Y, Imaoka S, Koyama H, Iwanaga T. Results of surgical treatment for sternal metastasis of breast cancer. Cancer. 1988. 62:1397–1401.

3. Brower ST, Weinberg H, Tartter PI, Camunas J. Chest wall resection for locally recurrent breast cancer: indications, technique, and results. J Surg Oncol. 1992. 49:189–195.

4. Park HM, Tarver RD. Solitary sternal metastasis from breast carcinoma. Clin Nucl Med. 1983. 8:373–374.

5. Veronesi G, Scanagatta P, Goldhirsch A, Rietjens M, Colleoni M, Pelosi G, et al. Results of chest wall resection for recurrent or locally advanced breast malignancies. Breast. 2007. 16:297–302.

6. Lequaglie C, Massone PP, Giudice G, Conti B. Analysis and long-term survival in sternectomy with plastic reconstruction for primary and secondary neoplasms of the sternum. Chir Ital. 2001. 53:485–494.
7. Lequaglie C, Massone PB, Giudice G, Conti B. Gold standard for sternectomies and plastic reconstructions after resections for primary or secondary sternal neoplasms. Ann Surg Oncol. 2002. 9:472–479.

8. Arnold PG, Pairolero PC. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg. 1996. 98:804–810.

9. Halm HF, Hoffmann C, Winkelmann W. The use of a Gore-Tex soft-tissue patch to repair large full-thickness defects after subtotal sternectomy. A report of three cases. J Bone Joint Surg Am. 2001. 83A:420–423.
10. Wuisman P, Scheld H, Tjan T, Roessner A, Blasius S, Vestring T, et al. Chondromyxoid fibroma of the sternum. Case report. Arch Orthop Trauma Surg. 1993. 112:255–256.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download