Abstract
Purpose
To identify the influence of lymphedema on health-related quality of life (HRQOL) more than 1 year after breast cancer surgery.
Methods
Ninety-six breast cancer patients who survived more than 1 year after surgery and 104 members of the general population were recruited. Patients were divided into 2 groups according to the presence of lymphedema. HRQOL was evaluated with the Short-Form 36-Item Health Survey.
Results
There were no statistically significant differences in any scales between groups: groups of breast cancer survivors with and without lymphedema. Compared with the general population, breast cancer survivors had lower quality of life scores in all scales, although the vitality and mental health scales did not differ from chance variation at the 5% level.
Although lymphedema has been described for centuries, recently more attention has been paid to the disease due to its presence as a relatively common complication of treatment of malignancy [1]. Lymphedema may impact health-related quality of life (HRQOL) in several ways. This lymphedema can develop at any time from initial treatment to 20 years later and the prevalence varies from 0% to 56% [2]. Lymphedema causes a wide range of discomfort and disabilities, and can affect patients in physical, functional, occupational, psychosocial, cognitive, lifestyle, and financial dimensions [3-6]. Therefore, relationship between lymphedema and quality of life has emerged as an important component in caring for breast cancer survivors.
Many studies comparing the quality of life between the breast cancer survivors and general population have reported lower quality of life of the former. Depending on the period, breast cancer survivors reported poorer HRQOL scores than the general population at the time of diagnosis and a year later [7,8]. In a long-term follow-up study, survivors who developed recurrence of new primary breast cancer experienced worse quality of life in all domains except social functioning. When limited to multi-year survivors who remained free of disease, their quality of life was similar to that among controls, with the exception of arm problems and sexual satisfaction [9].
The aim of this study was followed: First, is there any difference of HRQOL between breast cancer survivor with lymphedema and without lymphedema? Second, is there any difference of HRQOL between patients with breast cancer and normal population?
We recruited breast cancer survivors who visited Kyungpook National University Hospital (KNUH) Breast Clinic from April 2009 to March 2011. They were medically stable at least 1 year after surgery and finished breast cancer treatment. All patients who were enrolled in this study had no diagnosis and treatment of lymphedema before. They filled out Short-Form 36-Item Health Survey (SF-36) questionnaire and were then approached to diagnose lymphedema. Some patients had clinical symptoms in the affected arm, while some did not. Patients with impaired cognitive function or coexisting arm morbidities due to previous fracture or surgery were excluded. Individuals who had breast cancer surgery more than 10 years ago were also excluded to minimize recall bias. The normal population group was recruited from people who visited the KNUH health survey center for general health screening and had no history of breast cancer by a researcher. Among them, age-matched women were randomly selected by a doctor who was blinded to this study. This study protocol was reviewed and approved by KNUH Institutional Review Board (IRB approval No. 2010-10-020).
SF-36 questionnaire and then diagnosis of lymphedema was performed.
HRQOL has been used extensively in clinical and epidemiological research and hearth service studies [10]. Particularly, the Medical Outcome Study SF-36 is a widely used, generic, patient-reported, health status measure [11]. The SF-36 comprises eight health sub-scales (physical function, PF; role physical, RP; bodily pain, BP; general health, GH; Vitality, VT; social function, SF; role emotional, RE; and mental health, MH). Three scales (PF, RP, and BP) correlate most highly with the physical component and contribute most to the scoring of the Physical Component Summary (PCS) measure. The mental component most highly correlates with the MH, RE, and SF scales, which also contribute most to the scoring of the Mental Component Summary (MCS) measure. Three of the scales (VT, GH, and SF) have noteworthy correlations with both components [12]. In this study, we used the Korean version of SF-36 which has proven reliability and validity [13].
The presence of lymphedema was confirmed by one rehabilitation doctor based on history and physical examination. The lymphedema was determined by their arm circumference. This method is more practical in clinical settings, despite some limitations, principally reliability of inter- or intra-test [14]. Circumferential measurement of the 4 measurements points are at 10 cm above elbow crease, 7 cm below elbow crease, wrist, and mid-palm level. The contralateral arm circumference at corresponding levels was used as a reference to determine lymphedema. Lymphedema was defined as an increase in arm circumference at any level by 2 cm or more compared to the contralateral side. Severity of lymphedema was divided into 3 degrees (a difference in circumference up to 2 cm indicates mild lymphedema, a difference of 2 to 5 cm shows moderate lymphedema, and a difference of more than 5 cm is considered severe). Patients who had been diagnosed in this way underwent a lymphoscintigraphy scan. Lymphoscintigraphy is a relatively noninvasive technique involving an intradermal injection of radiolabeled colloid in the distal aspect of the edematous limb and imaging of the lymphatic vasculature [1]. The result was interpreted by one nuclear medicine doctor.
The SPSS version 13.0 (SPSS Inc., Chicago, USA) was used. In demographic data, age and duration after surgery were compared with independent t-tests. Characteristics except age were compared using the chi-square test. SF-36 scale scores were compared using the independent t-test. Null hypotheses of no difference were rejected if p-values were less than 0.05.
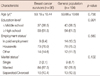
The ages of patients with lymphedema and without lymphedema were respectively 54.10±10.80 and 51.81±9.84 years. There were no statistically significant differences in age, education level, employment status, and a marital status. Among characteristics related to breast cancer including duration after surgery, there were have no statistically significant differences except surgery type. In categories of severity of lymphedema, mild lymphedema was most common. Among 58 lymphedema patients diagnosed by arm circumference, 32 patients were confirmed by lymphoscintigrapy as lymphedema (Table 1).
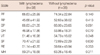
The ages of breast cancer survivors (n=97) and general populations (n=104) were respectively 53.19±10.44 and 53.66±10.68 years. There were no statistically significant differences in age, education level, employment status, and marital status (Table 2).
When comparing the difference between patients with lymphedema and without lymphedema, there were no statistically significant differences on all scales (Table 3).
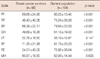
When comparing breast cancer survivors with the general population, there were statistically significant differences on all scales of SF-36 except VT and MH (Table 4).
The survival rate of breast cancer increased by early diagnosis, surgery, and development of adjuvant therapy. Survivors encounter many challenges throughout their lives because of complications. This is the reason for ongoing research in the HRQOL of breast cancer survivors. Upper limb lymphedema is one of the most burdensome complications of breast cancer treatment. It may significantly impair physical and psychological well-being of breast cancer survivors. Lymphedema causes deformity, functional disability, pain and recurrent infections within an edematous limb. It is also associated with psychological morbidity. Women with lymphedema experience anxiety, depression, social isolation and sexual problems [4,15-18]. Therefore, the relationship between lymphedema and quality of life has emerged as an important component in caring for breast cancer survivors.
In this study, when breast cancer survivors and the general population control group were compared by the SF-36, vitality and mental health did not differ statistically, but the value of all subscales were lower in the breast cancer survivor group. These two subscales are associated with the mental component of quality of life. This result is thought to be closely related to survivor period. As mentioned in the research of Pearman [19], quality of life typically improves after treatment for 6 to 12 months, then stabilizes. Therefore, in this study, patients over 1 year after breast cancer surgery might adapt to their illness and treatment and become mentally stable. This is in contrast to some studies regarding functional recovery of breast cancer that suggest general emotional and mental health is lower than other functions [8].
All patients participated in this study received counseling from psychiatric staff and assistance from a nurse for recovery of mental health during their admission for surgery. After discharge, the visits included an appointment with rehabilitation doctors, a physical examination, counseling, and appropriate medical intervention (except lymphedema treatment) if it was necessary. These series of actions is thought to have had a significant impact on stabilizing patients' mental health.
Many studies comparing the quality of life between the breast cancer survivors and general population have reported various results. Some studies reported that HRQOL scores of breast cancer survivors were lower than that of the general population at the time of diagnosis and after several years [7-9], while others find that the overall quality of life of breast cancer survivors 1 year after diagnosis is comparable to women from the general population [20].
In general, the physical component is more affected than mental component. Velanovich and Szymanski's study [5] showed that patients with lymphedema had significantly lower scores in the domains of role-emotional and bodily pain. In several other studies, patients with lymphedema have lower values in physical and functional quality of life than those without lymphedema, but no differences in emotional and mental health-related aspects [3,21]. However, those authors found that patients with lymphedema had lower quality of life scores except bodily pain component but there were no statistically significant differences. The results of this study that lymphedema associated with inferior physical component of quality of life, especially arm function, is similar to previous studies [3,22]. However, we noticed that there were no statistically significant differences.
For that reason, in our hospital, we conduct an intensive patient care system. After breast cancer surgery, patients had regular visits to the rehabilitation department. They have a chance to be educated about prevention and complications by a lymphedema therapist and be examined by a rehabilitation doctor. They inform that they have an opportunity to receive on early diagnosis, and of course, if it is necessary, they can receive early treatment. This multidisciplinary care might give patient psychological wellbeing and be responsible for the better prognosis of our patients compared to other studies.
There is another reason thought to be closely related to the survival of our patients. The period of a year is sufficient time to adapt to the disease and treatment in breast cancer patients regardless of the existence of lymphedema. Mak et al. [3] also mentioned women with lymphedema had time to adapt psychologically to this chronic problem, while those without lymphedema may have uncertainty and fear towards the potential impact of arm symptoms with which they had less experience.
There are some limitations in this study. First, this study is not a longitudinal study but cross-sectional. Further study about longitudinal change of quality of life in patients is needed. Because we recruited patients after a year, we cannot compare the quality of life at diagnosis or early treatment period. Thus, in the future, prospective studies from the time of diagnosis of breast cancer including breast cancer treatment and complications will be needed. Also, in this lymphedema patient group, further study related treatment result of lymphedema might be meaningful. Another limitation is small sample size. In the future, large-scale studies need to be designed that statistical limitations may not happened.
In this study, the presence of lymphedema in breast cancer patients who survived over 1 year after surgery might not affect the quality of life. However quality of life of breast cancer survivors is lower than in general population except for some mental health components.
Figures and Tables
Table 1
Characteristics of breast cancer survivors: with lymphedema vs. without lymphedema

BMI=body mass index; MRM=modified radical mastectomy; BCT=breast-conserving therapy; LN=lymph node.
*Values are expressed as means±SD; †Values are expressed as number (%); ‡Grade of lymphedema was divided by a difference of circumference. In this study, mild lymphedema means only a difference of 2 cm.
References
1. Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: a comprehensive review. Ann Plast Surg. 2007. 59:464–472.
2. Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women's Health Study. J Clin Oncol. 2008. 26:5689–5696.

3. Mak SS, Mo KF, Suen JJ, Chan SL, Ma WL, Yeo W. Lymphedema and quality of life in Chinese women after treatment for breast cancer. Eur J Oncol Nurs. 2009. 13:110–115.

4. Voogd AC, Ververs JM, Vingerhoets AJ, Roumen RM, Coebergh JW, Crommelin MA. Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. Br J Surg. 2003. 90:76–81.

5. Velanovich V, Szymanski W. Quality of life of breast cancer patients with lymphedema. Am J Surg. 1999. 177:184–187.

6. Carter BJ. Women's experiences of lymphedema. Oncol Nurs Forum. 1997. 24:875–882.
7. Lee ES, Lee MK, Kim SH, Ro JS, Kang HS, Kim SW, et al. Health-related quality of life in survivors with breast cancer 1 year after diagnosis compared with the general population: a prospective cohort study. Ann Surg. 2011. 253:101–108.

8. Schou I, Ekeberg Ø, Sandvik L, Hjermstad MJ, Ruland CM. Multiple predictors of health-related quality of life in early stage breast cancer. Data from a year follow-up study compared with the general population. Qual Life Res. 2005. 14:1813–1823.

9. Dorval M, Maunsell E, Deschênes L, Brisson J, Mâsse B. Long-term quality of life after breast cancer: comparison of 8-year survivors with population controls. J Clin Oncol. 1998. 16:487–494.

10. Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 health survey for use in Japan. J Clin Epidemiol. 1998. 51:1037–1044.

11. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992. 30:473–483.
13. Han CW, Lee EJ, Iwaya T, Kataoka H, Kohzuki M. Development of the Korean version of short-form 36-item health survey: health related QOL of healthy elderly people and elderly patients in Korea. Tohoku J Exp Med. 2004. 203:189–194.

15. Pyszel A, Malyszczak K, Pyszel K, Andrzejak R, Szuba A. Disability, psychological distress and quality of life in breast cancer survivors with arm lymphedema. Lymphology. 2006. 39:185–192.
16. Chachaj A, Małyszczak K, Pyszel K, Lukas J, Tarkowski R, Pudełko M, et al. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology. 2010. 19:299–305.

17. McWayne J, Heiney SP. Psychologic and social sequelae of secondary lymphedema: a review. Cancer. 2005. 104:457–466.

18. Mozes M, Papa MZ, Karasik A, Reshef A, Adar R. The role of infection in post-mastectomy lymphedema. Surg Annu. 1982. 14:73–83.
19. Pearman T. Quality of life and psychosocial adjustment in gynecologic cancer survivors. Health Qual Life Outcomes. 2003. 1:33.
20. Arndt V, Merx H, Stürmer T, Stegmaier C, Ziegler H, Brenner H. Age-specific detriments to quality of life among breast cancer patients one year after diagnosis. Eur J Cancer. 2004. 40:673–680.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download