Abstract
Purpose
The impact of time of surgery based on the menstrual cycle is a controversial issue. Two decades after the first interest in this topic, a number of studies with conflicting results have not helped to resolve this problem. This study aimed to prospectively evaluate the impact of timing of surgery based on the menstrual cycle on survival rates of breast cancer patients, and various clinical and hormonal classifications of the menstrual cycle were compared in order to determine the phase of the menstrual cycle which showed the highest degree of surgical survival.
Methods
Premenopausal breast cancer patients treated with curative surgery between 1998 and 2002 were prospectively included in this study. Patients were divided into different groups according to the first day of their last menstrual cycle using three different classifications (clinical, Hrushesky, Badwe), and were also grouped according to their serum hormone levels. Serum levels of follicle stimulating hormone, luteinizing hormone, estrogen, and progesterone were measured on the day of surgery.
Results
Ninety patients were included in the study. Median follow-up time was 90 months. Nineteen patients (21.1%) had loco-regional recurrence and/or distant metastases while 12 patients (13.3%) died during follow-up. Five-year (78.6% vs. 90.6%) and 10-year (66.7% vs. 90.6%) disease-free survival (DFS) rates of patients in the clinically defined follicular phase were significantly decreased compared to luteal phase. On the other hand, hormonally determined phases of the menstrual cycle and grouping of patients according to clinical classifications did not show an impact on prognosis.
Estrogen plays a central role in the etiology of breast cancer and is a well known target for treatment. Serum estrogen levels in the perioperative period might have an effect on prognosis and this effect could be dependent on the timing of surgery in the menstrual cycle. However, the impact of timing of surgery in the menstrual cycle is still a controversial issue. Two decades after the first interest on this topic, previous studies with conflicting results did not help to resolve this problem. Since 1989, 35 studies including 9,665 patients were reported and 11 studies reached a conclusion that supports a negative impact of surgery during the follicular phase of the menstrual cycle [1]. Retrospective nature of the majority of the previous studies prevented the researchers from reaching a definitive conclusion on this topic. In this study, it is aimed to prospectively evaluate the impact of timing of surgery based on the menstrual cycle on survival of breast cancer patients. In addition, various clinical and hormonal classifications of the menstrual cycle reported in previous studies were compared to determine the most effective classification on survival.
Premenopausal breast cancer patients treated with curative surgery between 1998 and 2002 were prospectively included in this study. Patients with irregular menses, history of previous gynecologic operations, use of oral contraceptives, treatment with neoadjuvant chemotherapy, radiotherapy, or hormonotherapy, those who were pregnant or lactating were excluded from the study. The procedures followed in this study were in accordance with the Helsinki Declaration. Demographic characteristics of the patients and histopathological properties of the tumors were recorded.
In order to evaluate the impact of menstrual cycle timing of surgery on survival, patients were clinically divided into groups according to the first day of their last menstrual cycle using three different classifications [2-4]. Groups formed according to these classifications were as follows: 1) follicular (days 0-14) and luteal (days 15-28) (clinical), 2) perimenstrual (days 0-6 and 21+) and mid-cycle (days 7-20) (Hrushesky), 3) estrogen effect (days 3-12) and other days (Badwe). In order to group patients according to their hormonal status, serum levels of follicle stimulating hormone, luteinizing hormone (LH), estrogen, and progesterone were measured on the day of surgery only. Patients with estrogen levels >100 pg/mL and LH levels >10 mIU/mL were deemed to be in the ovulatory phase, whereas patients with progesterone levels >2.5 ng/mL were deemed to be in the luteal phase. The remaining patients were deemed to be in the follicular phase.
Patient's age, disease stage, tumor size and grade, axillary status, estrogen and progesterone receptor status, timing of surgery in the menstrual cycle, adjuvant chemotherapy, radiotherapy, and hormonotherapy were evaluated as possible prognostic factors affecting disease-free survival (DFS) and overall survival (OS).
Patients were followed up at 3-month intervals for the first 2 years and at 6-month intervals between 2 to 5 years. A thorough history was taken and physical examination, liver function tests and tumor markers were performed at every visit. Mammography and breast ultrasonography were performed annually. Further investigative studies were carried out according to the complaints of the patients. The time to local recurrence, distant metastases, and death were calculated from the day of initial surgery to the last follow-up, or to the occurrence of the relevant event.
Survival estimates were determined using the Kaplan-Meier method. Univariate analysis for prognostic factors affecting DFS and OS was performed with a log-rank test. Cox stepwise regression analysis was used to determine the independent prognostic factors affecting DFS and OS. The frequencies of different variables in two patient groups were compared with Pearson's chi-square or Fisher's exact tests, as appropriate. Statistical analyses were performed with SPSS version 15.0 statistical software package (SPSS Inc., Chicago, USA). All of the tests applied were two-tailed, and the level of significance was accepted as being significant when p<0.05.
Ninety female patients with a median age of 41 years (range, 24-52 years) were included in the study. Patients' clinical characteristics as well as the treatment methods are shown in Table 1. There were 58 patients (64%) in the clinically defined follicular phase and 32 patients (36%) in the clinically defined luteal phase, whereas the number of patients in hormonally defined follicular and luteal phases were 50 (56%) and 40 (44%), respectively. In addition, according to Hrushesky's classification, 49 patients (54%) were perimenstrual and 41 patients (46%) were in the mid-cycle phase. According to Badwe's classification, 47 patients (52%) were in the estrogen effect phase whereas 43 patients (48%) were not. The distribution of clinical and histopathological characteristics of patients of these groups is shown in Table 2. There were no statistically significant differences between groups regarding these parameters.
Median follow-up time of the patients was 90 months (range, 9-141 months). Nineteen patients (21.1%) had loco-regional recurrence and/or distant metastases while 12 patients (13.3%) died during follow-up. Five-year and 10-year DFS rates were 83.1% and 75.5%, respectively. DFS estimates of the patients according to groups are shown in Table 3. On the other hand, 5-year and 10-year OS rates were 88.2% and 85.5%, respectively. OS estimates of patients according to groups are shown in Table 4.
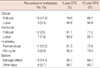
When the prognostic factors affecting DFS were evaluated, the 5-year (78.6% vs. 90.6%) and 10-year (66.7% vs. 90.6%) DFS of patients in the clinically defined follicular phase was less than those of the luteal phase. The difference between the two groups of patients was statistically significant (p<0.05) (Figure 1). Similarly, 5-year (81.7% vs. 84.9%) and 10-year (71.2% vs. 82.1%) DFS of the patients in hormonally defined follicular phase decreased compared to those of the luteal phase. However, the difference between the two groups of patients did not reach statistical significance (Figure 1). In addition, 5-year (85.3% vs. 81.3%) and 10-year (79.3% vs. 72.8%) DFS rates of the patients grouped as being in the mid-cycle phase according to the classification system of Hrushesky was higher compared to those in the perimenstrual phase, although this difference was not statistically significant (Figure 1). Patients who underwent operation in the estrogen effect phase according to Badwe had worse 5-year (78.6% vs. 90.6%) and 10-year (66.7% vs. 90.6%) DFS rates compared to those who underwent operation on other days, but the difference was not statistically significant (Figure 1).
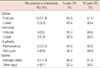
When the prognostic factors affecting OS were evaluated, 5-year (85.3% vs. 93.4%) and 10-year (81.0% vs. 93.4%) OS of patients in the clinically defined follicular phase was less than those of the luteal phase, however, the difference between the two groups of patients was not statistically significant (Figure 2). Similarly, 5-year (85.2% vs. 92%) and 10-year (80.6% vs. 92.0%) OS of patients in the hormonally defined follicular phase decreased compared to those of the luteal phase, though the difference between the two groups of patients was not determined to be statistically significant (Figure 2). In addition, 5-year (92.2% vs. 84.8%) and 10-year (88.8% vs. 82.6%) OS rates of patients grouped as being in the mid-cycle phase, according to the classification system of Hrushesky, was higher than those determined to be in the perimenstrual phase, although this difference was not statistically significant (Figure 2). Patients grouped in the estrogen effect phase according to Badwe had worse 5-year (86.8% vs. 90.0%) and 10-year (81.9% vs. 90.0%) OS rates compared to those undergoing operation on other days (Figure 2).
Besides the groups formed according to the phases of the menstrual cycle, various other factors were also found to have prognostic values (Table 5). Disease stage (p=0.040), tumor grade (p=0.006), and the presence of axillary metastases (p=0.020) had a significant effect on DFS. Similarly, disease stage (p=0.001), tumor grade (p=0.005), and the presence of axillary metastases (p=0.010) affected OS significantly. In contrast, hormone receptor status did not significantly affect either DFS or OS.
The relationship between time of surgery based on menstrual cycle and survival in breast cancer patients has been studied previously with conflicting results. Several studies supported the impact of time of surgery in the menstrual cycle and the results appeared to favor surgery in the luteal phase [2-7]. There are a few reasons for the conflicting results reported in previous studies. In the majority of these studies, information regarding patient's menstrual cycle was collected retrospectively from files or databases. Menstrual cycle data depending on patient records only is less valuable, since collecting this data is not the primary aim of the physician. In addition, only a few studies included an adequate number of patients and some of these studies were dependent on population based databases [8-12]. Finally, phases of the menstrual cycle were not standardized in previous studies. Menstrual cycles were divided in various different ways, which makes it difficult to compare these studies. Hormonal classification of the menstrual cycle was utilized in only one previous study [9]. The rather smaller numbers of patients included in the current study could be expected to affect the results. However, the current study has overcome a number of negative aspects of previous studies. In order to minimize the pitfalls in the methodology, patients in this study were included prospectively and data on the menstrual cycle was recorded on the day of surgery. Prospective design of the current study enabled us to depict a significant difference in DFS, which was in favor of performing surgery in the luteal phase. Additionally, patients were grouped clinically in several ways according to the phases of the menstrual cycle. In addition, serum hormone levels were used to support clinical data. Thus, previously reported clinical and hormonal classifications of the menstrual cycle were compared and clinical division of the menstrual cycle according to the first day of the last menstrual cycle into follicular and luteal phases was found to be the most effective system of grouping the patients. On the other hand, hormonally determined phases of the menstrual cycle did not show an impact on prognosis. When patients were classified hormonally rather than clinically, an 8% difference was detected in groupings, and this difference is the main reason for the statistically insignificant results obtained by comparison of these groups. Previously, a rate of 16% of misclassification was reported between menstrual cycle history and serum hormone levels for determining the exact phase of the menstrual cycle [13]. The use of selected hormone levels to determine the phases of the menstrual cycle might have a role in this disparity. Besides, grouping patients according to the classification systems of Hrushesky [2] and Badwe [4] was not found to have an impact on prognosis, although these classifications were previously reported to affect the prognosis.
Previous studies on the impact of time of surgery in the menstrual cycle had been based on the animal studies, which had implicated an easier spread of tumor cells to distant organs under conditions of elevated estrogen. Although the exact mechanisms playing a role in the outcome of surgery during the different phases of the menstrual cycle are not well known, several possible hypotheses have been posed. Since the impact of the time of surgery during the menstrual cycle is independent of hormone receptor status, hormones can be accepted to not have a direct effect on breast cancer progression [4,8]. The immune system should be the mediating mechanism between surgery, hormones of the menstrual cycle, and the spread of disease. Adrenergic stimulation occurring during surgery and anesthesia has a suppressive effect on the immune system, especially on natural killer cells. The immunosuppressive effect was shown to be more prominent in the follicular phase under the influence of estrogen in animal studies [14,15]. Suppression of the immune system in the perioperative period may accelerate the spread of tumor cells released into the circulation prior to or during surgery. This phenomenon may result in distant metastases decreasing the survival of the patients.
In a recent review, 35 previous studies including 9,665 patients were evaluated [1]. Almost all of the studies (33/35, 94.3%) were retrospectively designed. The time of surgery in the menstrual cycle was reported to have a prognostic effect within 15 studies (42.9%) including one prospective study [11]. Performing surgery in the follicular phase of the menstrual cycle was shown to have a negative prognostic impact in most of these studies (11/15, 73.3%).
Hrushesky et al. [2] reported the first clinical study on this topic in 1989. In this retrospective study, 44 breast cancer patients were evaluated, and patients having surgery in the perimenstrual phase had a decreased rate of survival compared to those operated on mid-cycle [2]. Similarly, Badwe et al. [4] studied the same topic, including 294 patients using a different classification of menstrual cycle phases. This study reported a worse 10-year survival for patients having surgery between days 3 to 12 when levels of estrogen were higher [4]. This effect was more prominent in patients with tumors <2 cm and axillary lymph node metastases and was independent of hormone receptor status [4].
Later, Veronesi et al. [8] reported a thoroughly analyzed series with a long follow-up time including 1,175 patients. Timing of surgery was found to be related to patient outcome in this study, and performing surgery in the luteal phase was found to prolong survival. This effect was independent of hormone receptor status and was restricted to node positive patients [8]. In addition, Kroman et al. [10] evaluated patients of a Danish national database in their study. This study, including 1,635 patients, contained the highest number of patients of any study. Timing of surgery in the menstrual cycle was reported not to have an impact on prognosis. However, the retrospective nature of data collection may have affected the results of this study as described above. In contrast to other previous studies, Pujol et al. [9] determined the patients' menstrual phase prospectively studying collected serum samples from 360 patients. It was assumed that hormonal classification of the patients would be more accurate than clinical classification in this study. However, timing of surgery in the menstrual cycle was reported to have no prognostic influence, after patient stratification according to lymph node status. Similarly, in the current study, hormonal classification of the patients was found to have no impact on survival.
In conclusion, in the current study patients were followed post surgery for more than 7 years, and performing surgery in the follicular phase (days 0-14) of the menstrual cycle showed a strong tendency to decrease DFS in premenopausal patients. As the follow-up time increased, a similar decrease in OS for patients operated under estrogen influence might be expected. According to these results, performing surgery during the luteal phase (days 15-28) of the menstrual cycle might have a beneficial effect on survival. Hence, we further conclude that surgery for breast cancer patients could be postponed until the luteal phase (days 15-28) of the menstrual cycle, where feasible. In addition, hormonotherapy might have a potential role in counteracting unopposed estrogen during the follicular phase in patients with hormone-dependent tumors. However, due to the rather smaller number of patients included in the study, the results should be considered with caution, and further studies which suitably appreciate the potential disparity in menstrual phase estimation, should be carried out.
Figures and Tables
 | Figure 1Disease-free survival plots of the patients grouped according to various phases of the menstrual cycle (A) clinical classification, (B) hormonal classification, (C) Hrushesky classification, (D) Badwe classification. |
 | Figure 2Overall survival plots of the patients grouped according to various phases of the menstrual cycle (A) clinical classification, (B) hormonal classification, (C) Hrushesky classification, (D) Badwe classification. |
References
1. Kroman N. Timing of breast cancer surgery in relation to the menstrual cycle: the rise and fall of a hypothesis. Acta Oncol. 2008. 47:576–579.

2. Hrushesky WJ, Bluming AZ, Gruber SA, Sothern RB. Menstrual influence on surgical cure of breast cancer. Lancet. 1989. 2:949–952.

3. Senie RT, Rosen PP, Rhodes P, Lesser ML. Timing of breast cancer excision during the menstrual cycle influences duration of disease-free survival. Ann Intern Med. 1991. 115:337–342.

4. Badwe RA, Gregory WM, Chaudary MA, Richards MA, Bentley AE, Rubens RD, et al. Timing of surgery during menstrual cycle and survival of premenopausal women with operable breast cancer. Lancet. 1991. 337:1261–1264.

5. Saad Z, Bramwell V, Duff J, Girotti M, Jory T, Heathcote G, et al. Timing of surgery in relation to the menstrual cycle in premenopausal women with operable breast cancer. Br J Surg. 1994. 81:217–220.

6. Saad Z, Vincent M, Bramwell V, Stitt L, Duff J, Girotti M, et al. Timing of surgery influences survival in receptor-negative as well as receptor-positive breast cancer. Eur J Cancer. 1994. 30A:1348–1352.

7. Hagen AA, Hrushesky WJ. Menstrual timing of breast cancer surgery. Am J Surg. 1998. 175:245–261.
8. Veronesi U, Luini A, Mariani L, Del Vecchio M, Alvez D, Andreoli C, et al. Effect of menstrual phase on surgical treatment of breast cancer. Lancet. 1994. 343:1545–1547.

9. Pujol P, Daures JP, Brouillet JP, Chang S, Rouanet P, Bringer J, et al. A prospective prognostic study of the hormonal milieu at the time of surgery in premenopausal breast carcinoma. Cancer. 2001. 91:1854–1861.

10. Kroman N, Højgaard A, Andersen KW, Graversen HP, Afzelius P, Lokdam A, et al. Danish Breast Cancer Cooperative Group. Timing of surgery in relation to menstrual cycle does not predict the prognosis in primary breast cancer. Eur J Surg Oncol. 1994. 20:430–435.

11. Love RR, Duc NB, Dinh NV, Shen TZ, Havighurst TC, Allred DC, et al. Mastectomy and oophorectomy by menstrual cycle phase in women with operable breast cancer. J Natl Cancer Inst. 2002. 94:662–669.

12. Goldhirsch A, Gelber RD, Castiglione M, O'Neill A, Thürlimann B, Rudenstam CM, et al. Menstrual cycle and timing of breast surgery in premenopausal node-positive breast cancer: results of the International Breast Cancer Study Group (IBCSG) Trial VI. Ann Oncol. 1997. 8:751–756.

13. Badwe RA, Wang DY, Gregory WM, Fentiman IS, Chaudary MA, Smith P, et al. Serum progesterone at the time of surgery and survival in women with premenopausal operable breast cancer. Eur J Cancer. 1994. 30A:445–448.

14. Goldfarb Y, Ben-Eliyahu S. Surgery as a risk factor for breast cancer recurrence and metastasis: mediating mechanisms and clinical prophylactic approaches. Breast Dis. 2006. 26:99–114.

15. Chaudhry A, Puntis ML, Gikas P, Mokbel K. Does the timing of breast cancer surgery in pre-menopausal women affect clinical outcome? An update. Int Semin Surg Oncol. 2006. 3:37.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download