INTRODUCTION
Cancer stem cells (CSCs) have recently been identified in various malignant tumors ranging from leukemia to solid tumors such as breast cancer. Evidence indicates that CSCs play an important role in the pathogenesis, invasion, and metastasis of malignancies [
1]. CSCs represent only a small fraction of a tumor. However, unlike most cancer cells, they possess self-renewal and differentiation capabilities, which lead to high tumorigenesis and resistance to standard chemotherapeutic agents. Breast cancer cells with the CD44
+/CD24
- cell surface marker expression profile have been proposed to be CSCs [
2]. Evidence has demonstrated that breast cancer cells with CD44
+/CD24
- have strong tumor initiation capabilities. Breast cancer cells with CD44
+/CD24
- are resistant to chemotherapy [
3] and radiotherapy [
4] because they are mostly quiescent, as they are arrested at the G0/G1 phase in mitosis. Inducing the breast cancer cells differentiation with CD44
+/CD24
- from G0/G1 to S may make these cells easy to destroy with chemotherapy.
We try to provide a strategy to induce breast cells differentiation with CD44+/CD24- from G0/G1 to S. Basic fibroblast growth factor (bFGF) is a widely active and potent mutagen. We propose the hypothesis that bFGF can be used to induce breast cancer cells with CD44+/CD24- to differentiate into dividing cancer cells.
Molecular markers have been used to identify breast CSCs, and the CD44
+/CD
24-/low phenotype has stem cell properties [
5]. A single breast cancer cell marked by CD44
+/CD24
- can reconstitute into a breast tumor [
6]. Breast cancer cells with CD44
+/CD24
- also have highly invasive properties and express higher levels of pro-invasive genes [
7].
In this study, we report that bFGF regulates progression of the G1/S phase, and a potential method to induce breast cancer
cells with CD44+/CD24- to distinguish cancer cells from MCF-7 cells.
Go to :

DISCUSSION
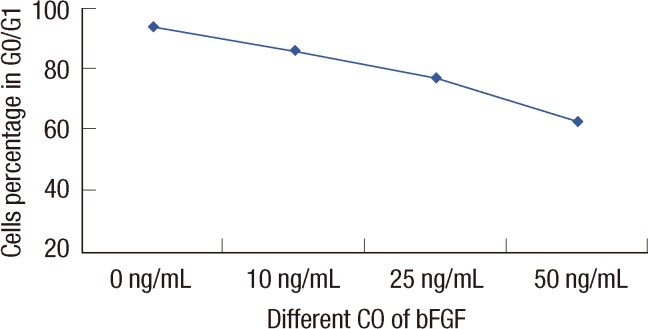
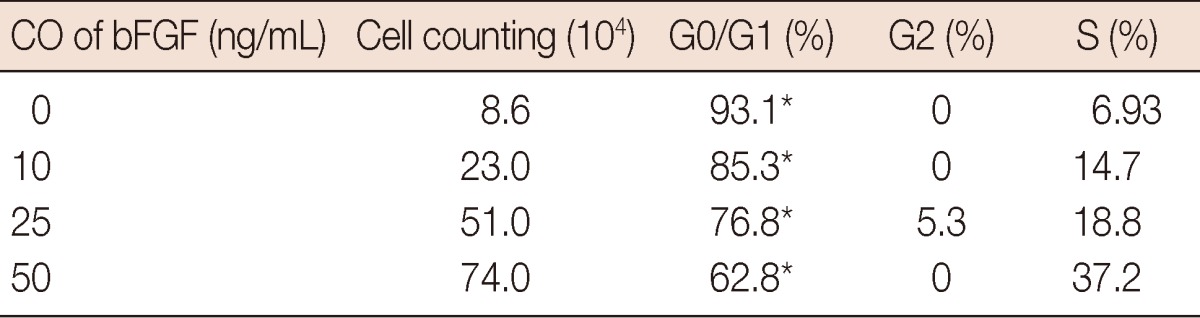
We demonstrated that the effect of bFGF on promoting CD44+/CD24-/low cell proliferation and progression through the G1/S phase transition was correlated with bFGF concentration.
FGF was first described in 1975 [
8]. Only acidic and basic FGF were identified, based on the structural properties of FGF. Today, FGF proteins are understood to comprise a growth factor family that includes more than 20 proteins [
9]. bFGF is mutagenic in various cell types, including bovine aortic endothelial cells [
10] and adult rat schwann cells [
11]; bFGF produces a 4-fold increase in DNA synthesis and a 3-fold rise in BrdU labeling, suggesting that it promotes the G1/S transition [
12]. When added to culture medium, the proportion of nifedipine-reactive patient cells that underwent progression to the S and G2/M phases from the G0/G1 phase increased significantly compared to the proportion in nifedipine-non-reactive patient cells [
13]; bFGF plays a concentration-dependent role regulating the cell cycle [
12,
14,
15]. The effect of bFGF in the regulation of the MCF-7 cell cycle with CD44
+/CD24
- was evaluated in this study, as bFGF is a potential factor promoting the G1/S transition.
Breast cancer cells that express CD44 (CD44
+) and show an absence or low levels of CD24
-/low possess self-renewal capacity and the ability to initiate tumors in non-obese diabetic/severe combined immunodeficient mice from as few as 100 cells [
16]. A recent study by Sheridan et al. [
16] also showed that CD44
+/CD24
-/low breast cancer cells possess enhanced invasive properties. Cancer cells with CD44
+/CD24
- differentiate into two types of cell during malignancy development one type retains self-renewal capacity, often arresting in G0/G1, whereas the other type loses self-renewal capacity and differentiates into a cancer cell. Stem cells are mainly quiescent in the G0 state [
14], which allows them to be drug-resistant. Some studies have shown that breast cancer cells with the CD44
+/CD24
- are more resistant to chemotherapy and radiotherapy than that of non-CSCs [
3,
4]. If we could promote cells to progress through the G1/S phase transition, breast cancer cell with CD44
+/CD24 might be more easily destroyed. As the number of breast cancer cell with CD44
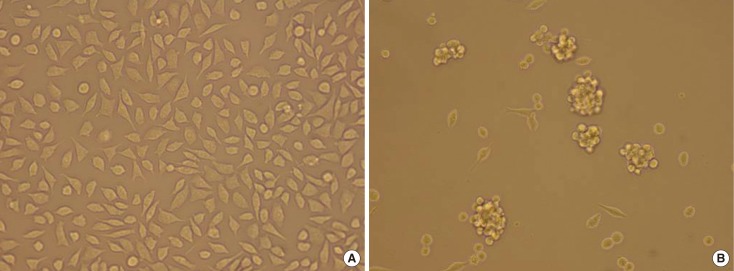
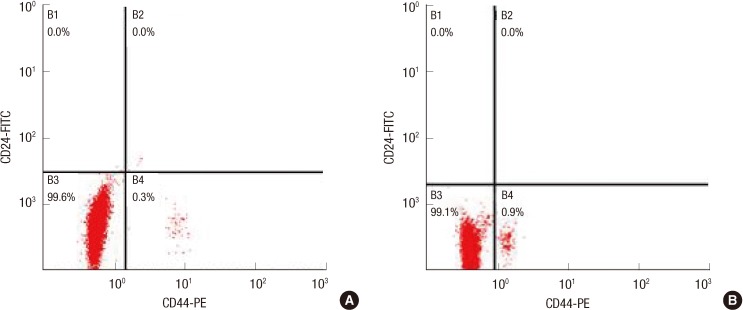
+/CD24 was very low, a mammosphere culture was used to obtain more cancer-initiating cells by adding 5 µg/mL bovine insulin, 20 ng/mL bFGF-2, 10 ng/mL epidermal growth factor and 2% B-27 into the DMEM-F12. Then, CD44
+/CD24
- cells were sorted by flow cytometry, and 0.9% breast cancer cells with CD44
+/CD24 were obtained from mammosphere culture, compared to 0.3% breast cancer cells with CD44
+/CD24 obtained from normal MCF-7. Grimshaw et al. [
17] found that mammosphere culture of pleural effusions enriched with CD44
+/CD24
low/- cells are capable of inducing tumors in severe combined immunodeficiency diseased mice.
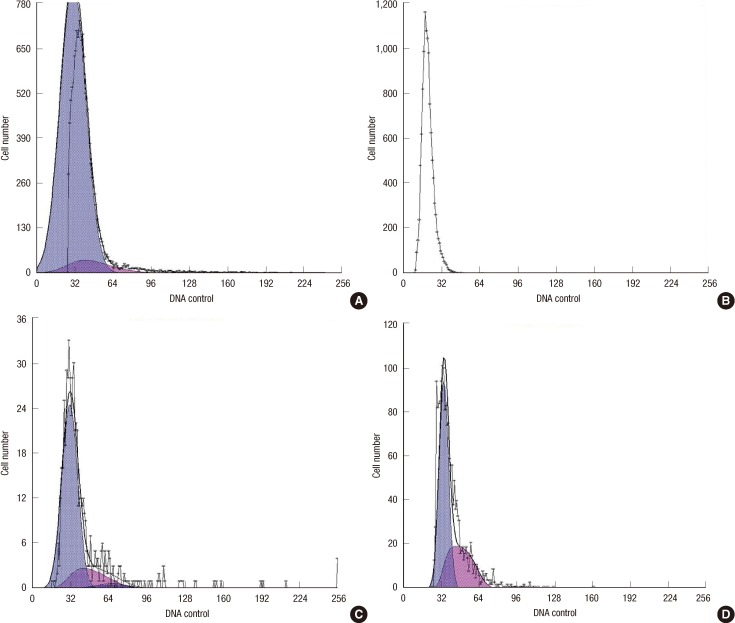
Cell cycle analyses were performed to calculate the percentage of CD44+/CD24- cells in G0/G1, under different bFGF concentrations. The results showed that the percentage of cells in G0/G1 decreased as bFGF concentration increased (p=0.023). The proportion was higher in the presence of a high level of bFGF than that with a in the relatively low level of bFGF. When bFGF was added to the medium, cell proliferation advanced in CD44+/CD24-/low cells as well as in MCF-7, cells without being selected. Cell count increased with increasing bFGF concentration.
In summary, the findings indicate that: First, mammosphere cultures were able to enrich CD44+/CD24- cells; Second, bFGF was able to sustain CD44+/CD24- cell proliferation and promote cell progression through the G1/S phase transition; Third, this effect was correlated with bFGF concentration.
However, the mechanism for the role of bFGF to enable breast cancer cells with the CD44+/CD24- to undergo progression to the S and G2/M phases from the G0/G1 phase is unclear, thus, further studies must be conducted. Promoting breast cancer cell with the CD44+/CD24- marker through the G0/G1→G2/S phase transition may be an efficient way to destroy breast cancer cells.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download