Abstract
Purpose
Amplification of the human epidermal growth factor receptor 2 (HER2) gene occurs in 18% to 20% of breast cancers, and it is recognized as a prognostic and predictive marker. We investigated the HER2 status in Korean breast cancer by immunohistochemistry (IHC) and silver-enhanced in situ hybridization (SISH), as the first step toward building a nationwide quality assurance program for HER2 testing.
Methods
A total of 1,198 breast carcinoma samples were collected from six institutions and IHC and SISH were performed using tissue microarrays in central laboratories. The results were compared to those of local laboratories.
Results
Available data were obtained from 959 samples. Central IHC results were negative, equivocal, and positive for 756 (78.8%; range among institutions, 76.8-81.8%), 37 (3.9%; 1.9-6.2%), and 166 (17.3%; 13.6-20%), respectively. SISH results were negative, equivocal, and positive for 756 (78.8%; 77.4-79.9%), 2 (0.2%; 0-0.7%), and 201 (21%; 20.1-22.2%), respectively. HER2 gene amplification was observed in 4.4%, 19%, and 73.9% of the negative, equivocal and positive groups stratified by local IHC results, respectively. When central SISH was considered to be the gold standard method for measuring HER2 status, the false-negative and false-positive rates of local IHC were 14.4% (29/201) and 7.1% (54/756). The concordance rate between central IHC and SISH was 98.4%.
Amplification of the human epidermal growth factor receptor 2 (HER2) gene and concomitant HER2 protein overexpression occur in approximately 18% to 20% of human breast cancers [1]. HER2 overexpression and/or gene amplification is associated with poor prognosis and has a strong predictive value for sensitivity to anthracycline-based chemotherapy and HER2-targeted therapies such as trastuzumab (Herceptin®; Genentech, South San Francisco, USA) or lapatinib (Tykerb®; GlaxoSmithKline, Philadelphia, USA) [2,3]. An accurate assessment of HER2 status is therefore important in the management of breast cancer patients, and HER2 status should be determined for all invasive breast cancers either at the time of diagnosis or at the time of recurrence [4].
Several methods have been developed to assess HER2 status in clinical samples. Because a fluorescence in situ hybridization (FISH)-based test was first approved as a diagnostic HER2 test by the United States Food and Drug Administration (FDA) in 1997, immunohistochemistry (IHC)-based diagnostic tests and brightfield in situ hybridization techniques, such as chromogenic in situ hybridization and silver-enhanced in situ hybridization (SISH), have been introduced to evaluate HER2 status. Although considerable debate exists regarding which test provides the best assessment of HER2 status [5], IHC has been widely used as the primary test for determining HER2 status, and samples with equivocal IHC results have been retested by FISH or SISH in Korea.
SISH is a new brightfield in situ hybridization method providing permanent signals that do not degrade and that are visible on a conventional brightfield microscope. SISH is performed using an automated immunostainer, the Benchmark® platform (Ventana Medical Systems, Tucson, USA). SISH is suitable for studies with large numbers of samples, and has exhibited high concordance with FISH for positive and negative results [6-8].
It is well known that IHC results are influenced by a variety of preanalytic (e.g., time to fixation, type and time of fixation, and method of tissue processing), analytic (e.g., type of antigen retrieval, test reagents), and postanalytic factors (e.g., interpretation criteria, quality assurance procedures) [1]. Earlier studies demonstrated that approximately 20% of HER2 assays performed in local laboratories had obtained incongruent results when the same specimens were retested in a high-volume, central laboratory [9,10]. The American Society of Clinical Oncology and College of American Pathologists (ASCO/CAP) has developed guidelines for optimal HER2 testing performance and have recommended an algorithm defining positive, equivocal, and negative results for both protein expression and gene amplification of HER2 [1]. The Breast Pathology Study Group of the Korean Society of Pathologists (BPSKP) has sought to build a nationwide program to accredit laboratories performing HER2 tests.
This study was designed to evaluate the incidence of HER2 gene amplification and its protein overexpression in invasive breast cancer in Korean patients, and to compare HER2 IHC results from local laboratories (local IHC) with those from the central laboratory, performed using standardized IHC (central IHC) and SISH procedures, for the purpose of collecting fundamental data to build a nationwide accreditation program for HER2 testing in Korea.
The BPSKP collected 1,198 invasive breast carcinoma samples from six university hospitals. Samples were collected from Asan Medical Center, University of Ulsan College of Medicine (216 samples, between January 1998 and December 1998), Chungnam National University Hospital (200 samples, between January 2000 and December 2003), Chonnam National UniUniversity Hwasun Hospital (204 samples, between January 1997 and December 2002), Soonchunhyang University Hospital (157 samples, between January 2000 and December 2003), Samsung Medical Center (199 samples, between January 2000 and December 2001), and Yeungnam University Hospital (222 samples, between January 2000 and December 2002) following approval of the local ethics committees (Asan 2011-0339 and YUMC PCR-11-35). All samples were formalin-fixed, paraffin-embedded, and processed in the routine diagnostic laboratory of each institution. A representative tumor block was selected by a pathologist affiliated with each institution. All blocks were submitted to the central laboratory (Asan Medical Center) to construct tissue microarrays (TMAs). Data regarding patient age at initial diagnosis, tumor size, histological type, histological tumor grade [11], lymph node status, surgery type, and local HER2 IHC results were also collected.
To minimize tissue loss and to compensate for tumor heterogeneity, three 1 mm cores were obtained from the most representative tumor area of each block, and were arrayed in a new recipient block using a manual TMA device (Beecher Instruments, Silver Springs, USA). In total, 56 TMA blocks were made from the 1,198 breast cancer samples. Using a standard microtome, 4 µm-thick sections were cut from TMA blocks, and were used to perform IHC (Yeungnam University Hospital) and SISH (Asan Medical Center) analyses.
IHC for HER2 protein was performed using a Benchmark® automatic immunostaining device (Ventana Medical Systems) according to the manufacturer's recommendations. Rabbit anti-HER2 antibody (CONFIRM anti-HER2/neu (4B5) rabbit monoclonal antibody; Ventana Medical Systems) and an UltraView™ universal DAB detection kit (Ventana Medical Systems) were used.
SISH was performed with INFORM® HER2 DNA and Chromosome 17 (Chr17) probes (Ventana Medical Systems) on two consecutive TMA sections using the Benchmark® automatic immunostaining device according to the manufacturer's protocol. Both probes were labeled with dinitrophenol (DNP). The HER2 DNA probe was denatured at 95℃ for 12 minutes, and hybridization was performed at 52℃ for 2 hours. After hybridization, an appropriate stringency wash (three times at 72℃) was performed. The Chr17 probe was denatured at 95℃ for 12 minutes, and hybridization was performed at 44℃ for 2 hours. After hybridization, appropriately stringent washes were performed three times at 59℃. The HER2 and Chr17 DNP-labeled probes were visualized using the rabbit anti-DNP primary antibody and the UltraView™ SISH Detection Kit. Silver precipitation was deposited in the nuclei following the sequential addition of silver acetate, hydroquinone, and H2O2, and the slides were then counterstained with Ventana hematoxylin II for interpretation by light microscopy.
Scoring of IHC results for HER2 was performed according to the ASCO/CAP guidelines [1]. A positive (3+) HER2 result was considered when the tumor exhibited uniform, intense membrane staining in more than 30% of the invasive tumor cells. An equivocal (2+) result was defined by weak or non-uniform, but complete membrane staining in more than 10% of the tumor cells, and a negative result was defined by weak incomplete membrane staining in any portion of the tumor cells (1+), or by the absence of any staining (0).
The SISH signals for HER2 and Chr17 were counted in more than 20 non-overlapping nuclei per sample. The evaluation was performed by one pathologist (Y.K.B.) using a conventional Nikon Eclipse 80i Microscope (×600 magnification) without knowledge of the HER2 results obtained by IHC. When intratumoral heterogeneity was observed in the signal distribution, more than 100 nuclei per sample were counted. A discrete dot was counted as a single copy of HER2 and Chr17. The size of these individual dots was used as a reference to determine the relative number of amplified copies in cancer cell nuclei. A small cluster of multiple signals was counted as six signals, and a large cluster was counted as 12 signals according to the manufacturer's instructions. As described in the ASCO/CAP guidelines [1], an absolute HER2 gene copy number >6 or a HER2/Chr 17 ratio >2.2 indicated SISH positivity; an absolute HER2 gene copy number between 4 and 6 or a HER2/Chr 17 ratio between 1.8 and 2.2, indicated SISH equivocalness; and an absolute HER2 gene copy number <4 or a HER2/Chr 17 ratio of <1.8 indicated SISH negativity. Lymphocytes, fibroblasts, and normal ductal epithelial cells were used as internal controls.
All statistical analyses were performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, USA). The concordance of data between central IHC and SISH and between local IHC and central IHC or SISH was calculated by cross-tabulation using Pearson chi-square test. False-positive and false-negative rates were calculated excluding equivocal (2+) samples that were clinically uninformative.
Of the 1,198 samples, we analyzed 959 (80%) samples for local IHC, central IHC, and SISH. The unused samples were excluded due to unavailable local IHC results, non-informative cores or the loss of cores while performing central IHC and SISH tests.
Of the 959 included samples, 276 patients underwent breast-conserving surgery, and 681 patients underwent mastectomy. Patient age ranged from 20 to 89 years (mean, 47.2 years). The histological types included invasive ductal carcinoma, not otherwise specified (832 samples, 86.7%), invasive tubular carcinoma (22 samples, 2.3%), mucinous carcinoma (15 samples, 1.6%), and others (90 samples, 9.4%). Tumor sizes varied from 0.5 to 13 cm (mean, 2.8 cm). Among 947 patients for whom primary tumor size data were available, 385 (40.7%), 498 (52.6%), and 64 (6.8%) tumors were categorized as pT1, pT2, and pT3, respectively. Of the 778 patients with available data, 384 (49.4%) patients had lymph node positivity at the time of surgery. The histological grade was available for 922 samples: 122 (13.2%) were grade 1, 408 (44.3%) were grade 2, and 392 (42.5%) were grade 3. The patient characteristics are summarized in Table 1.
Local IHC results for HER2 were described as negative (0 or -), focal or weak positive (+ or 1+), moderate positive (++ or 2+), or positive (1, +++, or 3+). We did not review local IHC slides, but for samples which were described as negative or focal, weak positive results comprised the HER2-negative group, whereas those described as moderate positive comprised the equivocal group, and those described as positive comprised the HER2-positive group. HER2 positivity rates varied among institutions (Figure 1A). Of the 959 samples, negative, equivocal and positive IHC results were observed for 664 (69.2%; range among institutions, 32.3-88.1%), 84 (8.8%; 0-36.4%), and 211 (20.8%; 11.9-31.3%) samples, respectively.
Central IHC results were negative, equivocal, and positive for 756 (78.8%; range among institutions, 76.8-81.8%), 37 (3.9%; 1.9-6.2%), 166 (17.3%; 13.6-20%) samples, respectively.
SISH results were negative, equivocal, and positive for 756 (78.8%; 77.4-79.9%), 2 (0.2%; 0-0.7%), and 201 (21%; 20.1-22.2%) samples, respectively (Table 2, Figure 1B, C).
The variability of the positive, equivocal, and negative rates of central IHC and SISH according to institutions was lower than that of local IHC (Figure 1A-C). The results of local and central IHC were consistent for 854 samples, with a concordance rate of 89.1%. Of the 211 samples with a local IHC result of 3+, 140 (66.4%) samples had a positive central IHC result. In the equivocal (2+) and negative (0-1+) groups for local IHC, 10 (11.9%) and 16 (2.4%) samples, respectively, were positive in central IHC (Table 2).
We compared the local IHC results with those of central SISH. The results for both methods were consistent in 95.5% of samples with negative (0/1+) IHC results and 73.9% of samples with positive (3+) IHC results. When samples with equivocal (2+) results were excluded, the concordance rate between local IHC and central SISH was 90.3%. HER2 amplification was detected by SISH in 156 (73.9%), 16 (19%), and 29 (4.4%) samples with positive, equivocal, and negative local IHC results, respectively (Table 2). When central SISH was considered the gold standard, the false-negative and false-positive rates of local IHC were 14.4% (29/201) and 7.1% (54/756), respectively.
The central IHC and SISH results were consistent for 908 samples, with a concordance rate of 94.7% (Table 3). HER2 amplification was observed in 1.7%, 62.2%, and 99.4% of the IHC-negative, equivocal, and positive groups, respectively. When we excluded samples with equivocal IHC results, the concordance rate between the two methods was 98.4%. There were discordant results for 14 samples including 13 IHC-negative/SISH-positive samples and 1 IHC-positive/SISH-negative sample (Figure 2).
Because HER2 gene amplification is directly linked to protein expression levels in breast cancers, IHC for HER2 protein expression and ISH for HER2 gene amplification can substitute for each other. DNA is less affected by tissue processing artifacts, and experimental errors occur less frequently during HER2 gene assessment by ISH. However, IHC results can be affected by a number of critical factors including tissue processing (preanalytic factors), antigen retrieval methods, the type of reagents and primary antibodies (analytic factors), and subjective interpretation of the staining result (postanalytic factors) [5].
We performed IHC and SISH with the current IHC methodology on TMA specimens (1997-2003) collected from six university medical centers in Korea and compared their results to those reported by the original institution at the time of the diagnosis, without knowing what the IHC methodology or reagents used by these institutions and reviewing of the original IHC slides. The interlaboratory variability of the central IHC results was markedly decreased compared to that of local IHC. The percentage of equivocal samples was more than 50% lower for central IHC than for local IHC (3.9% vs. 8.8%). The incidence of equivocal samples for central IHC coincided with that of an earlier study using the same method as our study (TMA slides, 4B5 monoclonal antibody, automated assay; 3.9% vs. 2-4%, respectively) [12].
Several factors may have contributed to the discrepancy between the local and central results. During the years between 1997 and 2003, the commonly available antibodies for HER2 were either rabbit polyclonal or mouse monoclonal antibodies, which are not directly comparable to the rabbit monoclonal antibody used in this study. We performed HER2 tests on TMA slides in this study (central IHC and SISH), however, the original institution performed HER2 test in the whole tissue sections at the time of the diagnosis (local IHC). Therefore, intratumoral heterogeneity of HER status may contribute to the discrepancy between local and central results. Lastly, the current more standardized IHC protocol and interpretation (ASCO/CAP guidelines, central IHC and SISH) vs. unknown IHC protocol and criteria (pre ASCO/CAP, local IHC) is thought to be further reason for the discrepancy between local and central results. The CAP/ASCO guidelines for HER2 testing became available in 2007 [1], and most laboratories have streamlined procedures to conform to these guidelines, thus becoming more concordant.
The ASCO/CAP recommends that a laboratory performing HER2 IHC must have concordance testing with an alternative validated method, and the finding of 95% or more samples being classified into either positive or negative categories supports the validated assay [1]. Equivocal samples are not expected to be concordant; and should be tested by another confirmatory analysis. The laboratories participating in the present study did not satisfy this criterion because the concordance rate between local IHC and central SISH was 90.3% for samples with positive or negative IHC results (82.4% concordance for all samples). In addition, the false-negative and false-positive rates of local IHC were 14.4% and 7.1%, respectively. Most of the local IHC results were obtained at the time of diagnosis (between 1997 and 2003) of primary breast carcinomas, and trastuzumab was not frequently prescribed in Korea at that time because the FDA did not approve trastuzumab for the treatment of HER2-positive breast cancer in the adjuvant setting until 2006 [3]. Therefore, the possibility of inappropriate treatment decisions for anti-HER2 targeted therapy would be low.
As the guidelines for HER2 testing were published by ASCO/CAP, pathology laboratories rapidly developed the capacity to assay HER2 status by adhering to these guidelines. For example, in a previous study, the false-positive rate of HER2 IHC was decreased from 27% in 2002 to 14% in 2009 [13]. In recently published Korean studies, the concordance rates between IHC and SISH were found to be 98.5 and 98.3% when samples with equivocal IHC results were excluded [8,14]. Therefore, we would expect a high concordance rate between local IHC and central SISH results if we were to collect more recent samples from local laboratories.
The incidence of HER2-positive tumors among primary breast carcinomas is expected to be 18% to 20%. Among 959 samples in our study, HER2 overexpression was observed in 17.3% of samples, and HER2 amplification by SISH analysis was observed in 21% of samples. A similar incidence of HER2-positive tumors was observed in a recently published Korean study performed in a single institution. Park et al. [15] reported HER2 overexpression and HER2 gene amplification by FISH in 16.8% and 24.2% of 950 Korean patients with invasive breast cancers, respectively. These incidence rates could be used as a reference for HER2-positive rates in establishing a nationwide program as a quality assurance measure for HER2 testing in Korea. A nationwide ISH program in Australia reported a gradual reduction of HER2 positivity in primary breast cancer for the period of October 2006 to September 2008. The HER2-positive rate was 23.8% in the first 12 months, and it decreased to 16.9% in the second 12 months; additionally, the proportion of IHC 3+ samples identified by ISH was increased from 80.2% to 84.4% in the second 12 months [16]. The authors concluded that the reduced HER2 positivity rate reflects improvements in testing accuracy due to increasing laboratory experience and the growing number of tests performed.
In conclusion, central IHC and SISH markedly decreased the interlaboratory variability of HER2 status compared with local IHC. Measurements of HER2 gene amplification by SISH were less affected by preanalytic factors than measuring HER2 protein overexpression by IHC. However, a number of laboratories in Korea use IHC as the first-line test in conjunction with ISH as a confirmatory test to assess HER2 status. Validation of IHC is necessary by all laboratories as a requirement of the ASCO/CAP guidelines. The central IHC and SISH results were highly concordant in this study, but the variability of IHC results in local laboratories stressed the need for constant vigilance to ensure accurate HER2 testing. The quality control program for HER2 testing needs to be focused on decreasing both the false negativity and false positivity of IHC in local laboratories. Nationwide quality control and assurance programs to validate IHC and ISH are being developed in Korea.
Figures and Tables
 | Figure 1Local immunohistochemistry (IHC) (A), central IHC (B), and silver-enhanced in situ hybridization (C) results by institution. |
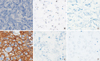 | Figure 2Representative examples of discordant results between immunohistochemistry and silver-enhanced in situ hybridization (SISH). One case exhibited (A) negative (0) HER2 immunohistochemistry, but HER2 gene amplification (B) was observed when compared with the chromosome 17 (Chr17) signals (C) in SISH analysis. Another case exhibited (D) positive (3+) HER2 immunohistochemistry, but SISH data were negative for HER2 amplification (E, F). The HER2/Chr17 ratio was 1 (×400). |
References
1. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007. 25:118–145.

2. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001. 344:783–792.

3. Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009. 14:320–368.

4. Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007. 25:5287–5312.

5. Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol. 2009. 27:1323–1333.

6. Dietel M, Ellis IO, Höfler H, Kreipe H, Moch H, Dankof A, et al. Comparison of automated silver enhanced in situ hybridisation (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists. Virchows Arch. 2007. 451:19–25.

7. Kang J, Kwon GY, Lee YH, Gong G. Comparison of silver-enhanced in situ hybridization and fluorescence in situ hybridization for HER2 gene status in breast carcinomas. J Breast Cancer. 2009. 12:235–240.

8. Sung WJ, Park SJ, Gu MJ, Bae YK. Automated silver-enhanced in situ hybridization for evaluation of HER2 gene status in breast carcinoma: comparison with fluorescence in situ hybridization and immunohistochemistry. Korean J Pathol. 2010. 44:28–34.

9. Paik S, Bryant J, Tan-Chiu E, Romond E, Hiller W, Park K, et al. Real-world performance of HER2 testing: National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst. 2002. 94:852–854.

10. Roche PC, Suman VJ, Jenkins RB, Davidson NE, Martino S, Kaufman PA, et al. Concordance between local and central laboratory HER2 testing in the breast intergroup trial N9831. J Natl Cancer Inst. 2002. 94:855–857.

11. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991. 19:403–410.

12. van der Vegt B, de Bock GH, Bart J, Zwartjes NG, Wesseling J. Validation of the 4B5 rabbit monoclonal antibody in determining Her2/neu status in breast cancer. Mod Pathol. 2009. 22:879–886.

13. De P, Smith BR, Leyland-Jones B. Human epidermal growth factor receptor 2 testing: where are we? J Clin Oncol. 2010. 28:4289–4292.

14. Park K, Han S, Kim JY, Kim HJ, Kwon JE, Gwak G. Silver-enhanced in situ hybridization as an alternative to fluorescence in situ hybridization for assaying HER2 amplification in clinical breast cancer. J Breast Cancer. 2011. 14:276–282.

15. Park S, Park HS, Koo JS, Yang WI, Kim SI, Park BW. Breast cancers presenting luminal B subtype features show higher discordant human epidermal growth factor receptor 2 results between immunohistochemistry and fluorescence in situ hybridization. Cancer. 2012. 118:914–923.

16. Bilous M, Farshid G. Assessment of human epidermal growth factor receptor 2 (HER2) amplification by in situ hybridization (ISH): findings from a nationwide program in Australia. 32nd Annual CTRC-AACR San Antonio Breast Cancer Symposium. 2009. Abstract #5100.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download