Abstract
We describe two cases of post-radiation sarcoma after breast cancer treatment. The first patient was a 61-year-old woman who underwent partial mastectomy of the right breast and adjuvant whole breast irradiation 7 years previously. Subsequently, a rapidly growing mass from the anterior arc of the right fifth rib was incidentally detected on an abdomino-pelvic computed tomography scan. The second patient was a 70-year-old woman who received neoadjuvant chemotherapy and a partial mastectomy of the left breast 9 years ago. Adjuvant irradiation was delivered to the whole breast and supraclavicular region. Subsequently, an approximate 8 cm mass developed in the left axillary area. Both patients received wide excision of the tumor with negative resection margins. The pathological diagnoses were osteosarcoma and undifferentiated pleomorphic sarcoma, respectively. Although post-radiation sarcomas are rare complications with a poor prognosis, enhanced awareness and early detection by clinicians are essential to improve outcomes via curative surgical resection.
Post-radiation sarcomas are rare complications of radiotherapy (RT) that occur within a previously irradiated field after several years of latency [1]. Since 1948, the criteria suggested by Cahan et al. [1] have been the diagnostic basis for post-radiation bone sarcoma. In 1999, Murray et al. [2] proposed revised criteria including the following: 1) The radiation must have been given previously, and the sarcoma that subsequently developed must have arisen in the area included within the 5% isodose line; 2) No evidence that the sarcoma was likely to have been present before the onset of irradiation; 3) All sarcomas must be proven histologically and must clearly be of a different pathology than that of the primary condition.
Post-radiation sarcoma accounts for 0.5% to 5.5% of all sarcomas [3]. Osteosarcoma, malignant fibrous histiocytoma, and fibrosarcoma are the most common histological subtypes [2,3]. Because adjuvant RT after breast-conserving surgery plays a significant role in the treatment of early-stage breast cancer [4], sarcomas of the breast, chest wall, sternum, axilla, or supraclavicular region have been reported as a rare complication of RT for breast cancer [5-7]. In total, 1,831 cases of radiation-induced sarcoma of the breast have been published in the English literature [8]. However, only a few cases of post-radiation osteosarcoma after RT for breast cancer have been reported in Korea. Here, we report two additional cases of post-radiation sarcoma after breast cancer treatment: osteosarcoma at the rib and undifferentiated pleomorphic sarcoma at the axilla.
A 61-year-old woman was referred to the Department of Thoracic Surgery with a rapidly growing chest wall mass. She complained of mild chest wall pain. She underwent a partial mastectomy of her right upper inner breast and a sentinel lymph node biopsy 7 years previously. The tumor was a 0.9×0.8 cm tubular carcinoma with a low nuclear grade, and lymph node metastasis was absent. The resection margins were all negative, with a minimum distance of 0.6 cm. Immunohistochemistry was positive for the estrogen receptor (ER) and progesterone receptor (PR). One month later, she received adjuvant RT for 6 weeks. The dose delivered to the whole breast was 50 Gy in 25 fractions using tangential fields of 4 MV photons (Figure 1), and the boost dose to the primary tumor bed was 10 Gy in five fractions using a 9 MeV electron beam. Adjuvant RT was well-tolerated, and no specific complications were observed during RT. After completion of RT, she took toremifene for 5 years.
She has undergone outpatient follow-up for a right ovarian cyst at the Department of Gynecology for the past 5 years. An abdomino-pelvic computed tomography (CT) scan was performed 1 month ago to evaluate the characteristics of the right ovarian tumor. A rapidly growing tumor from the anterior arc of the right fifth rib was incidentally detected on a CT scan (Figure 2A). The tumor was not seen on a CT scan performed 4 months previously. The tumor was hypermetabolic (Figure 2B) on positron emission tomography (PET), and a whole body bone scan demonstrated increased radio-uptake (Figure 2C). Ultrasound-guided core biopsy results showed a malignant mesenchymal tumor with osteoid deposition.
She underwent wide excision of the chest wall, including the fourth to sixth ribs. The pathological diagnosis of the chest wall mass was osteosarcoma of French Federation of Cancer Centers Sarcoma Group (FNCLCC) grade 3 (Figure 3). Resection margins were all negative.
A 70-year-old woman was referred to the Department of Orthopedic Surgery for a left axillary mass. She had been diagnosed with cancer of the left breast 9 years previously. She had received three cycles of neoadjuvant chemotherapy consisting of adriamycin and taxol for treatment of inflammatory breast cancer and ipsilateral axillary lymphadenopathy. She then underwent a partial mastectomy and axillary dissection. The tumor was a 1.4×1.4 cm high nuclear grade infiltrating ductal carcinoma. Extensive intraductal carcinoma and lymph node metastasis were absent, and the minimum resection margin was 0.5 cm. The tumor was ER and PR negative, but positive for human epidermal growth factor receptor 2. Adjuvant irradiation was delivered to the whole breast and ipsilateral supraclavicular region with a dose of 50 Gy in 25 fractions. The ipsilateral breast was treated with tangential fields of 4 MV photons, and the supraclavicular area was treated with an anterior oblique field of 4 MV photons (Figure 4). The primary tumor bed was boosted at a dose of 12 Gy in five fractions using a 9 MeV electron beam.
Chest CT and magnetic resonance imaging scans showed an approximate 8 cm mass with peripheral enhancement and pectoralis major muscle invasion in the left axillary area (Figure 5A, B). The tumor was hypermetabolic on the PET scan (Figure 5C). Focal fluorodeoxyglucose uptake was observed in the right thyroid gland. The result of a fine needle aspiration was papillary thyroid carcinoma.
She underwent wide excision of the axillary mass, and the tumor was an undifferentiated pleomorphic sarcoma of FNCLCC grade 3 (Figure 6). Resection margins were negative, but very close to the tumor. She is scheduled to receive surgical resection of the thyroid carcinoma.
Two cases of post-radiation sarcoma after breast cancer treatment were described. The first case developed within a previously irradiated field, and the second case developed at the margin of the irradiated field. The latent periods were 7 and 9 years, respectively. Because >5% of the prescribed dose had been delivered to the axillary area and there was no evidence of tumor on a previous PET scan, the second case also fulfilled the revised criteria suggested by Murray et al. [2]. Both patients underwent wide excision of the tumor with negative resection margins. The patients characteristics and those of a previously reported case are shown in Table 1.
It is interesting that papillary carcinoma was detected in the right thyroid gland of the second patient, which had been included within the 5% isodose line. But there was an insufficient imaging study covering the thyroid gland. Therapeutic radiation for a childhood malignancy has been associated with malignant thyroid neoplasms [9]. But a population based, retrospective cohort study regarding the risk of thyroid carcinoma after RT for breast cancer showed no significant increase in the risk of thyroid carcinoma in either the RT cohort or the non-RT cohort compared with that in the general population [10]. Taken together, the causal relationship between RT and thyroid carcinoma cannot be determined.
As survival after breast cancer treatment is typically long-term, the risk of secondary malignancies, particularly sarcomas, increases. According to a large retrospective study of 16,705 patients treated for breast cancer, adjuvant RT significantly increases the rate of sarcomas and lung cancers when compared with a non-RT group (p=0.020 and p=0.022, respectively) [11]. Among them, 35 patients developed sarcomas, 27 of whom fulfilled Cahan's criteria [6]. The cumulative incidence of radiation-induced sarcoma was 0.27% and 0.48% at 10 and 15 years, respectively. Compared with the general population, the standardized incidence ratio is 10.2% and 1.3% for patients with breast cancer who did or did not receive RT, respectively. Another study using surveillance, epidemiology, and end result data reported the cumulative incidence of sarcoma at 15 years as 0.32% for cases receiving RT, which was more frequent than that of cases that did not receive RT (0.23%, p=0.001) [5]. The mean latency periods of the above two studies were 8.7 and 7.5 years, respectively, which was similar to our two cases. Angiosarcoma is the most prevalent histology among radiation-induced sarcomas [5,6], though our cases were not of this type (Table 1).
Higher radiation dose increases the risk for soft tissue and bone sarcomas after breast cancer [12]. Compared with patients who receive <14 Gy, the odds ratios are 1.6 and 30.6 for patients who receive 14 to 44 Gy and >44 Gy at the site of the sarcoma, respectively. According to this finding, the first patient of the present report, who received 45 Gy at the site of sarcoma, was at high risk for developing post-radiation sarcoma (Table 1). Intensity modulated radiotherapy (IMRT) has been developed recently to improve target coverage and reduce normal tissue complication probability for breast cancer [13]. But, IMRT increases contralateral lung and breast volume exposed to low doses. Because the second case developed at the site exposed to a relatively low dose, a second cancer occurrence, including sarcoma following exposure to low dose radiation, should be considered.
There are no distinguishable imaging features of post-radiation sarcomas [3]. However, the presence of bony destruction with a soft tissue mass, tumor matrix mineralization at a previously irradiated area, and an appropriate latency period could be important clues for a diagnosis of post-radiation sarcoma. In addition to imaging features, a low incidence after a long latency period makes it difficult to diagnose the disease accurately. Thus, concern and awareness by clinicians is needed for an accurate diagnosis.
The prognosis of post-radiation sarcomas is generally poor, with 5-year survival rates of 27% to 36% [5-7]. The standard treatment is surgical resection, but this is often prohibited by tumor location [6,7,14]. Only complete surgical resection can guarantee long-term survival [14]. Chemotherapy or RT has limited roles in treatment. Therefore, early detection is important to enable curative resection.
Knowledge and experience regarding post-radiation sarcomas in Korea is limited to a few case reports. Despite well-organized nationwide breast cancer databases [15], no large-scale study has been conducted regarding post-radiation sarcomas after breast cancer treatment. Given the younger age at diagnosis and the increased use of RT as a primary treatment for breast cancer [4,15], longer survival after treatment could lead to more cases of post-radiation sarcoma. Future studies should investigate the incidence or distribution of sarcomas after RT for breast cancer based on analysis of a large database.
In summary, we report here two cases of post-radiation sarcoma after breast cancer treatment with latency periods of 7 and 9 years. Post-radiation sarcomas are a rare complication after such long latency periods. Curative surgical resection is the standard treatment, despite a poor prognosis. Enhanced clinician awareness and early detection are essential to improve clinical outcomes.
Figures and Tables
Figure 1
The previously used irradiation fields in case 1. Total doses delivered were 50, 45, and 40 Gy to the areas filled with red, blue, and pink, respectively. The fifth rib, where post-radiation sarcoma developed, is delineated in sky blue.
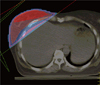
Figure 2
Imaging studies of case 1. An enhancing mass at the anterior arc of the right fifth rib was discovered on a computed tomography scan (A). The mass was hypermetabolic on a positron emission tomography scan (B) and increased radio-uptake was shown on a whole body bone scan (C).
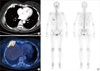
Figure 3
Histological examination of case 1. (A) Atypical tumor cells with osteoid production are shown on the specimen (H&E, ×200). The tumor revealed positive immunoreactivity for osteocalcin (B), cyclin-dependent kinase 4 (C), and Ki-67 (D, 60%) (immunohistochemical staining, ×200).
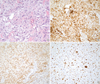
Figure 4
Coronal reconstruction of the previously used irradiation fields of case 2. The dose distributions of the filled areas are the same as those in Figure 1.
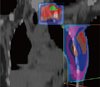
Figure 5
Imaging studies of case 2. An approximate 8-cm mass with peripheral enhancement developed in the left axillary area was observed on computed tomography (A) and magnetic resonance imaging scans (B). The mass was also hypermetabolic on a positron emission tomography scan (C).
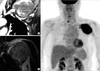
Figure 6
Histological examination of case 2. (A) Many atypical tumor cells were seen in the specimen (H&E, ×200). (B) The tumor had positive immunoreactivity for smooth muscle actin (immunohistochemical staining, ×200).

Table 1
Characteristics of post-radiation sarcoma after treatment of breast cancer in Korea

IDC=invasive ductal carcinoma; PM=partial mastectomy; SLNB=sentinel lymph node biopsy; ALND=axillary lymph node dissection; MRM=modified radical mastectomy; RT=radiotherapy; SCN=supraclavicular lymph node; ALN=axillary lymph node; IMN=internal mammary lymph node; MFH=ma-lignant fibrous histiocytoma.
*Previously reported case in Korean literature by Lim et al. (not referenced).
References
1. Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL. Sarcoma arising in irradiated bone: report of 11 cases. Cancer. 1948. 1:3–29.
2. Murray EM, Werner D, Greeff EA, Taylor DA. Postradiation sarcomas: 20 cases and a literature review. Int J Radiat Oncol Biol Phys. 1999. 45:951–961.

3. Sheppard DG, Libshitz HI. Post-radiation sarcomas: a review of the clinical and imaging features in 63 cases. Clin Radiol. 2001. 56:22–29.

4. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002. 347:1227–1232.

5. Yap J, Chuba PJ, Thomas R, Aref A, Lucas D, Severson RK, et al. Sarcoma as a second malignancy after treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2002. 52:1231–1237.

6. Kirova YM, Vilcoq JR, Asselain B, Sastre-Garau X, Fourquet A. Radiation-induced sarcomas after radiotherapy for breast carcinoma: a large-scale single-institution review. Cancer. 2005. 104:856–863.

7. Erel E, Vlachou E, Athanasiadou M, Hassan S, Chandrasekar CR, Peart F. Management of radiation-induced sarcomas in a tertiary referral centre: a review of 25 cases. Breast. 2010. 19:424–427.

8. Sheth GR, Cranmer LD, Smith BD, Grasso-Lebeau L, Lang JE. Radiation-induced sarcoma of the breast: a systematic review. Oncologist. 2012. 17:405–418.

9. Acharya S, Sarafoglou K, LaQuaglia M, Lindsley S, Gerald W, Wollner N, et al. Thyroid neoplasms after therapeutic radiation for malignancies during childhood or adolescence. Cancer. 2003. 97:2397–2403.

10. Huang J, Walker R, Groome PG, Shelley W, Mackillop WJ. Risk of thyroid carcinoma in a female population after radiotherapy for breast carcinoma. Cancer. 2001. 92:1411–1418.

11. Kirova YM, Gambotti L, De Rycke Y, Vilcoq JR, Asselain B, Fourquet A. Risk of second malignancies after adjuvant radiotherapy for breast cancer: a large-scale, single-institution review. Int J Radiat Oncol Biol Phys. 2007. 68:359–363.

12. Rubino C, Shamsaldin A, Lê MG, Labbé M, Guinebretière JM, Chavaudra J, et al. Radiation dose and risk of soft tissue and bone sarcoma after breast cancer treatment. Breast Cancer Res Treat. 2005. 89:277–288.

13. Krueger EA, Fraass BA, McShan DL, Marsh R, Pierce LJ. Potential gains for irradiation of chest wall and regional nodes with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2003. 56:1023–1037.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download