This article has been corrected. See "Erratum to: Ultrasonographic Characteristics of Mammographically Occult Small Breast Cancer" in Volume 15 on page 482.
Abstract
Purpose
To analyze significant ultrasonographic findings of small malignant breast mass (≤10 mm) which were occult on mammography.
Methods
The study included 190 small breast masses (≤10 mm), demonstrated on breast ultrasonography, but not mammography. Histopathology (when the masses were biopsied) or serial breast ultrasonography (for at least 24 months) were used to confirm benign or malignant condition of the masses. Univariate and multivariate logistic regression analysis were used to identify significant characteristic malignant findings on ultrasonography.
Results
Of 190 masses, 46 were cancer, and 144 were benign. On multivariate analyses, irregular shape (odds ratio [OR], 10.4) and not circumscribed margin (OR, 31.6) were significant features to differentiate between benign and malignant breast masses. However, low width/anteroposterior ratio, echogenic halo, hypoechogenecity and posterior acoustic shadow, which were predictors for malignancy in large breast mass, were not documented in small mass.
According to current American Cancer Society (ACS) Guideline, clinical breast examination (CBE) and mammography (MMG) are two modalities used for screening breast cancer in average risk women, not ultrasonography (US) [1,2].
CBE is a practical modality to detect early breast cancer and does not require any further technology. The efficacy of CBE can be improved when clinicians are trained with optimal technique [3]. A palpable abnormality is the most common presentation of breast cancer. However, small breast mass is unlikely to be detected during CBE.
MMG is a good radiological diagnostic modality in detecting breast cancer. However, a number of malignant breast masses, in particular in women who have dense mammographic density, are occult on MMG [4,5]. Although the use of additional US for breast cancer screening is not routinely recommended, its benefit in some particular women (i.e., women who have dense breast on mammographic density) is more widely accepted [6]. In Asian women, in whom dense breast tissue was commonly evidenced, malignant mass may be missed [7].
Advanced technology of high-resolution and automated whole breast US has demonstrated a benefit in detecting a small mass in the breast accurately [8]. Classical signs of a malignant mass, including irregular shape, not circumscribed margin, non parallel orientation and posterior acoustic shadow, can be identified clearer with the high-resolution machines. Thus, additional US may be beneficial in detecting a small breast mass [4,5,7,9,10], as well as aiding in obtaining tissue for diagnosis even for microcalcification [11].
To best of our knowledge, limited information documented the ultrasonographic findings and usefulness of clinical assessment on small mammographically occult malignant breast masses which were smaller than 10 mm. These masses, in clinical practice, are subtle on both clinical and ultrasonographic assessment, and more importantly, may not be able to differentiate between benign and malignance during performing US. This study, therefore, aimed to evaluate the significant malignant ultrasonographic findings which would probably be unique to small mammographically occult mass in the breast.
During 3-year period (2007-2009), 9,350 small breast masses (≤10 mm) in 3,667 patients, regardless screening or diagnosis and high risk or average risk women, were identified on US. Of these, 748 masses in 386 patients were detected in both MMG and US. Other 8,602 small breast masses in 3,281 consecutive patients were documented only on US, not MMG.
Of 8,602 masses documented on US, 130 had biopsy performed. According to pathological results, 46 were malignant and 84 were benign. Other 8,472 masses were observed with serial US for at least 24 months. In order to match the ratio of 1:3 (case:control) for further analysis, 46 malignant masses were grouped as case and 144 benign masses were selected as control. Amongst 144 benign masses, 84 had pathology proven and 60 had unchanged US features over at least 24 months follow-up period. The 60 unchanged US feature masses were randomly selected into the study using simple random sampling generated by computer program on PASW statistics 18 for Windows software (IBM, Somers, USA).
The study, finally, included 190 masses from 190 patients. The sonographic scan was conducted by experienced breast radiologists, using either LOGIQ 9, LOGIQ 700, or LOGIQ E 9 (General Electric Medical Systems, Milwaukee, USA) with 10 to 14 MHz linear-array probe. Advanced US technique with compound Speckle Reduction Imaging, Cross XBeam was used during all US examinations.
The mass was measured in two dimensions, anteroposterior (height) and width, with electronic calipers. At the time of examinations, ultrasonographic images were recorded as digital files in the PAC system EBM V.6.2.1245 (EBM Technologies, Honolulu, USA). The review processes were performed later on the high resolution computer screens for diagnostic radiology (5-million pixels). All ultrasonographic findings were retrospectively reviewed by two experienced diagnostic breast radiologists who worked in the Breast Imaging Center with more than 10 years experience in analysis of breast imaging. The two radiologists were blinded to histopathological results and were not the same radiologist who performed the examination. The review was performed individually, according to the guideline of the sonographic Breast Imaging Reporting and Data System (ACR BI-RADS-US Lexicon classification). The shape (oval, round irregular), orientation (parallel, not parallel), margin (circumscribed, not circumscribed), lesion boundary (abrupt interface, echogenic halo), echogenicity (anechoic, hyperechoic, complex, hypoechoic, isoechoic), posterior acoustic features (no, enhancement, shadowing, combined pattern), as well as surrounding tissue (duct change, Cooper's ligament changes, edema, architectural distortion, skin thickening, skin retraction/irregularity) were assessed and recorded. In this study, the mass was classified into not parallel orientation when the width/anteroposterior (W/AP) ratio of mass was <1.4 [12]. Any disagreement was subsequently reviewed concomitantly until a consensus was achieved.
Chi-square, multivariate logistic regression, odds ratio, and 95% confident interval (CI) of individual ultrasonographic features were used for statistical analysis. The p-value <0.05 was considered as statistically significance. All statistical analyses in the study were carried out using PASW statistics 18 for Windows software.
Of 8,602 small mammographically occult breast masses (≤10 mm) included in the study, 130 were biopsied. Forty-six were malignancy. Therefore, the biopsy rate was 1.5% and the positive biopsy rate was 35.4%. Average diameters for all malignant and benign masses were 6.8 and 7.0 mm, respectively.
One hundred and ninety masses included in the study were non-visualized on MMG, possibly due to high breast density documented the study population (Table 1). Of the 190 studied masses, 144 were benign and were grouped in the control group. Eighty-four were histologically confirmed their benign condition (33: fibroadenoma, 16: fibrocystic disease, 14: ductal hyperplasia, 7: papilloma, 4: sclerosing adenosis, 2: inflammation, 1: ductal ectasia, 1: fat necrosis, 1: foreign body reaction, and 1: phyllode tumor). As well, 60 masses with unchanged radiological findings over 24 months follow-up were regarded as benign, without pathological proven. In the case group, all 46 malignant masses had histological or cytological report (36: invasive ductal carcinoma, 5: in situ carcinoma, 3: invasive lobular carcinoma, 1: mucinous carcinoma, 1: invasive papillary carcinoma).
All masses in the study were classified according to BI-RADS classification. Of 190 masses; 102, 82, and 6 masses were BI-RADS 3, 4, and 5, respectively (Table 1). One mass classified into BI-RADS 3 was malignancy; none of BI-RADS 5 was benign. Thus, sensitivity, specificity, positive predictive value, negative predictive value and accuracy of breast US to differentiate benign from malignant masses were 98%, 70.6%, 51.1%, 99%, and 76.3%, respectively.
All ultrasonographic findings of 190 studied masses are demonstrated in Table 2. Irregular shape, not parallel orientation (W/AP ratio <1.4), not circumscribed margin, echogenic halo and posterior acoustic shadowing were sonographic signs significantly associated with malignancy (Table 2, Figure 1).
Furthermore, the significant ultrasonographic features were subjected to multivariate logistic regression analysis with malignancy as the dependent variable. Irregular shape and not circumscribed margin were significant variables in this model (Table 3).
Although, US is not routinely recommended by ACS as screening modality for breast cancer [1,2], its benefit in some women who had dense breast tissue were evidenced [5-7], in particular in detection of a small malignant breast mass [13]. Detection of small malignant mass is important in term of early breast cancer detection. As a result of wider use of US, incidental detection of subcentimeter benign breast masses was increasing [13,14]. However, diagnostic accuracy of sonography in detection of small malignant breast mass sized smaller than 10 mm is not clearly identified. Therefore, it is important to identify significant ultrasonographic signs used to distinguish benign and malignant breast mass sized smaller than 10 mm.
Finding from our study suggested that irregular shape and not circumscribed margin were ultrasonographic signs of small malignant breast mass (OR, 10.4 and 31.6, respectively). The findings were comparable to other previously reported data [10,14-17]. Other ultrasonographic signs previously documented in a large malignant breast mass, such as not parallel orientation, echogenic halo, hypoechoicity, posterior acoustic shadow may not be commonly demonstrated in small malignant breast mass.
Irregular shape is possibly a ultrasonographic sign of malignant breast mass [17]. Breast mass with oval or round shape is likely to be benign [17]. Finding from our study confirmed that irregular shape was a reliable sign of malignancy in small breast mass, less than 10 mm. The finding was contrary to a previous study where irregular shape was not a factor to differentiate between benign and malignant mass in tumors smaller than 10 mm, only not circumscribed margin was a significant factor [12]. This was possible due to use of recent advanced US technique in our study which may be able to eliminate unclear shape, comparing with the previous US machine without compound and coded harmonic modes used in the previous study.
According to ACR BI-RADS-US lexicon, not circumscribed margin is classified into indistinct, angular, lobulated and spiculated margin. Margin is the most reliable factor in differentiating benign and malignant amongst small breast masses [12]. Our study confirmed that indistinct margin was a significant ultrasonographic sign of malignancy. However, a spiculated margin, which was another reliable indicator for a malignant breast mass with highest positive predictive value of malignancy [10,12,15,16], had not been observed in our study. A spiculated margin is defined as imaging manifestation of infiltrative tentacles of tumors with extending to the surrounding tissue. A malignant tumor has inconsistent growth and ultimately presents as irregular shape [18]. However, the inconsistent growth in small tumors may start with minimal infiltration in all directions into surrounding normal hyperechoic breast tissue, thus, showing as irregular shape and indistinct margin in the early appearance. A spiculated margin may not be commonly documented in a small mass [10,12,15,16].
Other ultrasonographic signs used to discriminate benign and malignant breast mass demonstrated significant finding on univariate analysis, but not on multivariate logistic regression test were not parallel orientation, echogenic halo and posterior acoustic shadow.
Not parallel orientation (low W/AP ratio) was previously noted as a reliable ultrasonographic sign to differentiate between benign and malignant masses [19]. This sign is not only objective evidence on measurement but also demonstrate a high level of observer agreement [20,21]. However, cut-off point on W/AP ratio is inconclusive. Several studies used W/AP ratio less than 1 as a cut-off point, but relatively low sensitivity was observed. Other study suggested that W/AP ratio >1 was unreliable for masses less than 11 mm [22]. W/AP ratio <1.4 was the valuable threshold to distinguish fibroadenoma from cancer in a study where large numbers of small mass were included [18]. Our study could demonstrate a significant predictive value of W/AP ratio <1.4 in differentiating malignance from benign on the univariate analysis (OR, 2.77; p=0.004). However, there was still overlapping between our small benign and malignant masses when W/AP ratio <1.4 was observed. As a result, on multivariate logistic regression analysis W/AP ratio <1.4 could not represent a significant predictive factor for malignancy. Therefore, cut-off point of W/AP ratio to predict malignant process in tumor less than 10 mm is still inconclusive.
Lesion boundary is an important finding in differentiating benign and malignant masses in US examination. Echogenic halo is a sign of malignant mass in the lesion boundary criteria. Our study showed that echogenic halo was infrequent finding. Presence of echogenic halo in small breast mass demonstrated a raised concern for malignancy. The result was similar to the previous study where echogenic halo is an evidence supported malignant condition [17-19].
Findings in our study agreed with previous studies where internal echogenicity was not a good ultrasonographic sign for determining malignant property [4,12,16,20,23]. Small benign and malignant masses could show either hypoechogenicity or isoechogenicity (p=0.31). Although, marked hypoechogenicity is one of a malignant feature in large tumors [17], it was not a frequent sign in small mass.
Posterior shadowing is a frequent sign indicated a malignant behavior. A malignant mass tends to have a desmoplastic reaction and high tumor cellularity, comparing with benign mass. Thus, sound beam attenuation cannot penetrate through the mass [14]. Posterior shadowing is more frequently evidenced in low-grade malignancy and non-homogeneous tumors [17,19,24,25]. However, in a small mass, posterior acoustic shadowing, from the multivariate logistic regression analysis in this study, may not be a reliable sonographic sign of malignancy. A small mammographic occult mass is likely to have low desmoplastic reaction and less heterogeneous character, thus may not show posterior shadowing. Furthermore, we used compound imaging technique in this study. It is well known that posterior shadowing is diminished in this technique [26].
This study, however, had some limitations. First, not all benign masses in our study were proved by histopathology. Although, most masses which remain unchanged in size over 24-month period are benign, some slow progressive well-differentiated tumor can manifest with stable mass. Second, though, breast US examinations were reviewed by two breast radiologists, original ultrasound captions were performed by several breast radiologists who had varied experience, thus may cause interobserver variability. The review process was not on real-time images which might affect the decision of the radiologist. Finally, small number of malignant masses included in the study as a control group (46 masses) may affect statistical power of the study. Some common sonographic features such as spiculated margin, hypoechogenicity, and surrounding ductal dilatation were not documented following the analysis. Further study in a larger population is still required.
Based on US, irregular shape and not circumscribed margin were strong predictive signs of malignancy in small malignant breast mass. Meanwhile, presence of W/AP ratio ≤1.4, echogenic halo and posterior acoustic shadowing may not be commonly visualized in the small lesions. These findings will be useful for breast radiologists who will use the information to discriminate between benign and malignant lesions during performing breast US, in particular in the small breast masses.
Figures and Tables
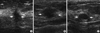 | Figure 1Ultrasonographic findings of malignant and benign breast masses. (A) A mass of invasive ductal carcinoma with irregular shape, not circumscribed margin and hypoechoicity (arrows). (B) A mass of invasive ductal carcinoma with oval shape, not circumscribed margin and hypoechoicity (arrows). (C) A mass, confirmed to be fibroadenoma, showed an oval shape mass, circumscribed margin and hypoechoicity (arrows). |
Table 1
Mammographic density and BI-RADS classification of 46 nodules in the case group and 144 nodules in the control group

References
1. Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA. 2005. 293:1245–1256.

2. Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010. 60:99–119.

3. Stojadinovic A, Summers TA, Eberhardt J, Cerussi A, Grundfest W, Peterson CM, et al. Consensus recommendations for advancing breast cancer: risk identification and screening in ethnically diverse younger women. J Cancer. 2011. 2:210–227.

4. Chao TC, Chen MF, Wang CS, Jan YY, Hwang TL, Chen SC. Small invasive breast carcinomas in Taiwanese women. Ann Surg Oncol. 2003. 10:740–747.

5. Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol. 2003. 181:177–182.

6. Corsetti V, Houssami N, Ghirardi M, Ferrari A, Speziani M, Bellarosa S, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011. 47:1021–1026.

7. Muttarak M, Pojchamarnwiputh S, Chaiwun B. Breast carcinomas: why are they missed? Singapore Med J. 2006. 47:851–857.
8. Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010. 20:734–742.

9. Bassett LW, Ysrael M, Gold RH, Ysrael C. Usefulness of mammography and sonography in women less than 35 years of age. Radiology. 1991. 180:831–835.

10. Moy L, Slanetz PJ, Moore R, Satija S, Yeh ED, McCarthy KA, et al. Specificity of mammography and US in the evaluation of a palpable abnormality: retrospective review. Radiology. 2002. 225:176–181.

11. Yu PC, Lee YW, Chou FF, Wu SC, Huang CC, Ng SH, et al. Clustered microcalcifications of intermediate concern detected on digital mammography: ultrasound assessment. Breast. 2011. 20:495–500.

12. Chen SC, Cheung YC, Su CH, Chen MF, Hwang TL, Hsueh S. Analysis of sonographic features for the differentiation of benign and malignant breast tumors of different sizes. Ultrasound Obstet Gynecol. 2004. 23:188–193.

13. Bae MS, Han W, Koo HR, Cho N, Chang JM, Yi A, et al. Characteristics of breast cancers detected by ultrasound screening in women with negative mammograms. Cancer Sci. 2011. 102:1862–1867.

14. Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995. 196:123–134.

15. Barton MB, Elmore JG, Fletcher SW. Breast symptoms among women enrolled in a health maintenance organization: frequency, evaluation, and outcome. Ann Intern Med. 1999. 130:651–657.

16. Chao TC, Lo YF, Chen SC, Chen MF. Prospective sonographic study of 3093 breast tumors. J Ultrasound Med. 1999. 18:363–370.

17. Skaane P, Engedal K. Analysis of sonographic features in the differentiation of fibroadenoma and invasive ductal carcinoma. AJR Am J Roentgenol. 1998. 170:109–114.

18. Hong AS, Rosen EL, Soo MS, Baker JA. BI-RADS for sonography: positive and negative predictive values of sonographic features. AJR Am J Roentgenol. 2005. 184:1260–1265.

19. Paulinelli RR, Freitas-Júnior R, Moreira MA, Moraes VA, Bernardes-Júnior JR, Vidal Cda S, et al. Risk of malignancy in solid breast nodules according to their sonographic features. J Ultrasound Med. 2005. 24:635–641.

20. Burnside ES, Hall TJ, Sommer AM, Hesley GK, Sisney GA, Svensson WE, et al. Differentiating benign from malignant solid breast masses with US strain imaging. Radiology. 2007. 245:401–410.

21. Rahbar G, Sie AC, Hansen GC, Prince JS, Melany ML, Reynolds HE, et al. Benign versus malignant solid breast masses: US differentiation. Radiology. 1999. 213:889–894.

22. Fornage BD, Lorigan JG, Andry E. Fibroadenoma of the breast: sonographic appearance. Radiology. 1989. 172:671–675.

23. Baker JA, Kornguth PJ, Soo MS, Walsh R, Mengoni P. Sonography of solid breast lesions: observer variability of lesion description and assessment. AJR Am J Roentgenol. 1999. 172:1621–1625.

24. Cole-Beuglet C, Soriano RZ, Kurtz AB, Goldberg BB. Ultrasound analysis of 104 primary breast carcinomas classified according to histopathologic type. Radiology. 1983. 147:191–196.

25. Kossoff G. Causes of shadowing in breast sonography. Ultrasound Med Biol. 1988. 14:Suppl 1. 211–215.

26. Kwak JY, Kim EK, You JK, Oh KK. Variable breast conditions: comparison of conventional and real-time compound ultrasonography. J Ultrasound Med. 2004. 23:85–96.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download