Abstract
Purpose
Invasive pleomorphic lobular carcinoma (IPLC) is a very rare and distinct morphological variant of invasive lobular carcinoma (ILC), characterized by nuclear atypia and pleomorphism contrasted with the cytologic uniformity of ILC. This study evaluated clinicopathologic characteristics and prognosis of IPLC compared with invasive ductal carcinoma (IDC).
Methods
We retrospectively reviewed the medical records of 35 patients with IPLC and 6,184 patients with IDC, not otherwise specified. We compared the clinicopathologic characteristics, relapse-free survival (RFS) and disease specific survival (DSS) of patients who were surgically treated between January 1997 and December 2010.
Results
Patients with IPLC presented at an older age with larger tumor size, worse histologic grade, higher rates of N3 stage, more multifocal/multicentric tumors, and more nipple-areolar complex involvement than those of patients with IDC. During the follow-up period, the IPLC group experienced five cases (14.3%) of disease recurrence and three cases (8.6%) of disease specific mortality compared with 637 cases (10.4%) of recurrence and 333 cases (5.4%) of disease specific mortality in the IDC group. Univariate analysis using the Kaplan-Meier method revealed that the IPLC group showed a significantly poorer prognosis than that of the IDC group (RFS, p=0.008; DSS, p<0.001). However, after adjusting for clinicopathologic factors, a multivariate analysis showed no statistical differences in RFS (p=0.396) and DSS (p=0.168) between the IPLC and the IDC groups.
Conclusion
Our data suggest that patients with IPLC present with poor prognostic factors such as large tumor size, poor histologic grade and advanced stage at diagnosis. These aggressive clinicopathologic characteristics may result in poor clinical outcomes. Although our study could not link IPLC histology to poor prognosis, considering the aggressive characteristics of IPLC, early detection and considerate treatment, including proper surgical and adjuvant intervention, could be helpful for disease progression and survival.
Invasive breast carcinoma is subdivided into ductal, lobular, tubular, and other special types by morphological classification. With the development of immunohistological and molecular biology techniques, invasive breast carcinoma has been further classified into new subtypes with unique characteristics and behaviors. Invasive pleomorphic lobular carcinoma (IPLC) is one such distinctive subtype of invasive breast carcinoma.
IPLC was first described in 1987 by Page and Anderson [1], and histological features were consolidated by Eusebi et al. [2] and Weidner and Semple [3] IPLC is very rare (less than 1% of all invasive breast carcinomas) and described as a variant of invasive lobular carcinoma (ILC). IPLC retains some characteristics of ILC, such as loss of E-cadherin expression [4], targetoid arrangement of tumor cells around the terminal duct and diffuse infiltration of tumor cells arranged in single files [5,6]. The distinguishable histologic features of IPLC include alveolar, solid, and mixed patterns of growth in the same tumor, enlarged nuclei, nuclear contour irregularity, increased hyperchromasia, and abundant eosinophilic and faint granular cytoplasm (Figure 1) [1,5-7].
IPLC has been reported with poor prognostic factors, including large tumor size, axillary node metastasis, poor histologic grade, and loss of E-cadherin. This aggressive biology may be reflected in the poor clinical course of IPLC, which has been noted in several studies to include a short relapse period, high risk of recurrence, and decreased survival [2,3,7,8]. In an extreme case, Eusebi et al. [2] reported a 60% mortality rate within 42 months of diagnosis. Some studies compared the clinical factors and prognosis of IPLC with those of invasive ductal carcinoma (IDC) or classic ILC and reported a worse prognosis for IPLC. However, due to the rare incidence of IPLC, these studies are limited by small sample size and short follow-up period. Thus, defining the clinical behavior and determining the proper management for IPLC is challenging.
For this reason, we present a retrospective analysis on our experience with IPLC, comparing clinicopathologic characteristics and prognosis with those of IDC to evaluate clinical behavior and proper management strategies for IPLC.
Patients were selected from the electronic database of Samsung Medical Center, Seoul, Korea between January 1997 and December 2010. Eligible patients included women who had undergone surgery for either IDC not otherwise specified (IDC NOS) or IPLC. Patients who had undergone pre-operative chemotherapy and/or radiation treatment or who presented with distant metastasis at the initial diagnosis were excluded. We identified 6,184 patients with IDC NOS and 251 patients with ILC patients. Among 251 ILC patients, 35 patients (14.0%) were classified as IPLC.
Data were extracted for the following variables: patient age, presence of multifocal/multicentric tumors, histologic grade, nipple-areolar complex (NAC) invasion, lymphovascular invasion, hormone receptor expression, and tumor, node, metastasis (TNM) stage according to the seventh edition of the American Joint Committee on Cancer (AJCC) staging manual [9]. Treatment modalities, such as type of operation, use of radiation therapy and, use of chemotherapy or hormone therapy were also evaluated. Disease-specific survival (DSS) was measured as the time from breast cancer diagnosis until death as a result of a breast cancer-related cause or until the date of last follow-up.
Estrogen receptor status of tumor specimens was determined by standard immunohistochemistry. Tumors were considered receptor-positive if they scored equal to or higher than 3+ on the Allred scoring system [10,11]. An Allred score ranges from 0 to 8, which combines a proportion score, range from 0 to 5 and an intensity score, ranging 0 to 3. Epidermal growth factor receptor 2 (HER2) status was determined on tumor tissues using anti-HER2 polyclonal antibody and tissues were considered receptor positive if the staining intensity was 3+. Scores of zero or 1+ were negative and scores of 2+ were further evaluated with fluorescence in situ hybridization (FISH).
Descriptive analyses were performed to explore clinicopathologic characteristics and treatment methods according to tumor type. Binomial analysis was assessed using either Pearson's χ2 test or the Mann-Whitney test. The linear-by-liner association test was used to assess for the presence of associations between IDC and IPLC. We used the Kaplan-Meier method and the log-rank test to compare relapse-free survival (RFS) and DSS between the two groups. Multivariate analysis was performed using the Cox-hazard regression method.
Data were analyzed using Microsoft Excel 2010 (Microsoft, Redmond, USA). Statistical analyses were performed using PASW Statistics 18.0 (SPSS Inc., Chicago, USA). Reported p-values are two-sided and statistically significant when p<0.05.
This retrospective cohort study included 6,184 patients with IDC NOS and 35 patients with IPLC. Clinicopathologic characteristics and treatment patterns are shown in Table 1. The mean follow-up periods in the IDC and IPLC groups were 46.5±36.5 and 36.3±27.4 months, respectively. Mean age at diagnosis was 47.8±9.9 years for patients in the IDC group and 51.4±10.5 years for patients in the IPLC group (p=0.033). The IPLC group presented with a significantly higher histologic grade (p=0.031) and larger tumor size than those in the IDC group (mean size, 3.2±1.8 cm in the IPLC group and 2.2±1.5 cm in the IDC group, p<0.001). The IPLC group tended to have more axillary nodal metastasis (mean, 3.9±8.0) than that in the IDC group (mean, 1.9±4.5, p=0.784). According to the seventh AJCC staging system, a high proportion of patients with IPLC was included as N3 (6 of 35 patients, 17.1%, p=0.010), and patients with IPLC were classified at higher stages than those with IDC (p=0.022). IPLC tumors were found more frequently at NAC (p=0.009) and were more likely to have multiple lesions (p=0.009). Consequently, the IPLC group showed an increased tendency to require a total mastectomy compared to the IDC group (p=0.084).
The IPLC group tended to express more estrogen receptor compared with the patients in the IDC group (77.1% vs. 69.5%, p=0.329), and more patients tended to undergo hormone therapy in the IPLC group than in the IDC group (85.3% vs. 73.6%, p=0.170). No significant differences were observed for the number of patients who underwent radiation treatment or chemotherapy (p=0.832 and p=0.161). The frequency of HER2 overexpression was not also statistically different between IPLC and IDC (28.6% vs. 25.2%, p=0.696).
During the mean follow-up of 36 months, five patients (14.3%) in the IPLC group experienced disease recurrence, and three patients (8.6%) experienced disease-specific mortality. Four cases of distant metastasis occurred in the IPLC group, including one case of both bone and liver metastases and one case each of bone, lung, and ovarian metastasis. In the IDC group, 637 patients (10.4%) experienced disease recurrence and 333 (5.4%) experienced disease-specific mortality (Table 2).
Figure 2 illustrates the Kaplan-Meier curves for RFS and DSS in both the IPLC and IDC groups. The IPLC group showed significantly worse prognosis than that in IDC group using the log-rank test (p=0.008 for RFS, p<0.001 for DSS). However, after adjusting for clinicopathologic factors, such as age, tumor size, nodal status, histologic grade, lymphovascular invasion, resection margin, hormone status and use of chemotherapy, Cox-Hazard regression analysis revealed no statistically significant differences in RFS (p=0.396) and DSS (p=0.168) between the two groups (Figure 3).
Because IPLC is a very rare subtype of invasive breast carcinoma, previous studies of IPLC were limited by a small number of cases and short follow-up times. As a result, it has been difficult to understand the clinical behavior, prognosis and treatment strategies for IPLC. Most studies have demonstrated that patients with IPLC often present with poor prognostic factors at diagnosis and that this is associated with an aggressive clinical course. However, it has not been determined whether this clinical course is a result of poor prognostic factors or IPLC histology itself. We compared RFS and DFS using univariate and multivariate analyses after adjusting for clinicopathologic factors to study these factors.
In our study, the IPLC group was associated with older age, larger tumor size, higher histologic grade and a more multifocal/multicentric location than those in the IDC group. Mean patient age at IPLC diagnosis was greater than that of patients with IDC, which was similar to the findings of previous reports [7,12]. Generally, aging is a major risk factor for the development of new breast cancer [13] and an aggressive prognostic factor [14,15]. However, the mean ages at diagnosis of patients with IPLC and IDC patients in our study were younger than those found in previous studies. Mean age at diagnosis of patients with IDC was 47.8 years and that of patients with IPLC was 51.4 years in this study compared with 56 years for patients with of IDC and 59 years for patients with IPLC in Buchanan et al. [7]. This age difference between Korean patients and Western patients may arise from ethnic differences. Recent statistics have shown that the peak age group for Korean patients with breast cancer is 45 to 49 years, and that more than half of all patients with breast cancer are diagnosed at under 50 years old [16].
Most studies have found that IPLCs are usually large at the time of diagnosis [5,7,8,12,17]. The mean IPLC tumor size in our study was 3.2±1.8 cm, whereas the mean IDC tumor size was 2.2±1.5 cm. The large size of IPLCSs at diagnosis may result from a rapid growth rate. Our data and those of prior studies [7,12] show that patients with IPLC have more poorly differentiated tumors compared with those in patients with IDC. A poor histologic grade, which reflects a high number of mitotic figures, strongly correlates with poor prognostic factors, including tumor size, vascular invasion, recurrence, and distant metastasis [18,19]. IPLC is generally associated with a higher incidence of axillary lymph node involvement than that of IDC [7,8]. In our study, patients with IPLC tended to have more axillary lymph node metastasis than that of patients with IDC, but the difference was not statistical significant. Interestingly, IPLCs were more often classified as N3 than IDCs (17.1% vs. 5.3%, p=0.010). In conjunction with large tumor size, a high prevalence of the N3 stage results in higher AJCC staging of IPLCs (p=0.022). Our data also indicate the IPLCs were more likely to invade the NAC and be multifocal/multicentric than IDCs. Consequently, there was a tendency that IPLC patients were more often treated with mastectomy than IDC patients (p=0.084).
The frequency of HER2 overexpression in IPLC has had variably reported values. Middleton et al. [20] reported that 81% of IPLCs show membranous staining for the HER2 receptor (2+ to 3+ by immunohistochemistry), whereas Jacobs et al. [6] reported that none of their patients with IPLC expressed HER2 staining by immunohistochemistry. In our study, 10 of the 35 IPLCs (28.6%) expressed a 3+ score on HER2 immunohistochemical staining. The correlation between HER2 overexpression and histologic grade is also controversial. Frolik et al. [21] reported that HER2 protein overexpression was detected in 53% of grade 3 IPLC tumors, whereas Varga et al. [12] reported that 5 out of 16 (31%) grade 3 IPLCs showed HER2 amplification in a FISH assay. In our study, 3 of 6 (50%) grade 3 IPLCs expressed a 3+ score on HER2 immunohistochemical staining without statistical significance (p=0.108).
Previous studies have reported that IPLCs show more aggressive characteristics compared not only with IDC but with classic ILC. In a study of Buchanan et al. [7], IPLCs were larger tumors, with more frequent lymphovascular and lymph node invasion than those in ILCs. Additionally, the recurrence rate of IPLC was significantly higher than that of ILC. Jacobs et al. [6] also reported that PLC is more frequently a higher grade and exhibits an adverse biomarker profile, such as loss of estrogen receptor expression and high Ki-67 expression compared with those of classic ILC.
These aggressive clinicopathologic characteristics of IPLCs may be reflected in aggressive clinical outcomes. Most studies have suggested that patients with IPLC have a worse prognosis than that of patients with IDC [2,3,7,8] and our Kaplan-Meier analysis result also demonstrated a similar finding. However, these findings do not conclude that IPLC itself is an aggressive biologic phenotype. Therefore, we adjusted the clinicopathologic characteristics and compared RFS and DSS using a multivariate analysis. The results failed to demonstrate that IPLC itself affects prognosis. Patients with IPLC were more likely to have estrogen receptor-positive tumors and be treated with hormone therapy compared with patients with IDC, although the difference was not significant. In general, IPLCs tend to express hormone receptors [20-22]. Decreased RFS and DSS due to aggressive clinicopathologic characteristics could support the routine use of chemotherapy and hormone therapy in patients with hormone receptor-positive tumors.
In summary, IPLC is very rare and patients present with poor prognostic factors such as large tumor size, high histologic grade, and advanced AJCC stage at diagnosis. These aggressive clinicopathologic characteristics may result in poor clinical outcomes. Although our study did not identify the contribution of IPLC histology to the aggressive prognosis, patients with IPLC showed worse clinical outcomes than that of patients with IDC. Given these characteristics of IPLC, early detection and considerate treatment, including proper surgical treatment, adjuvant chemotherapy, radiation therapy and/or hormonal treatment may be helpful in slowing disease progression and increasing survival.
Figures and Tables
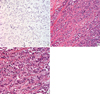 | Figure 1Microscopic morphology of invasive pleomorphic lobular carcinoma (IPLC). (A) IPLC image with E-cadherin staining (×400). Neoplastic cells were typically negative for E-cadherin staining. (B) IPLC image with H&E staining (×200). Note the diffuse infiltration of dissociated cancer cells arranged in single file. (C) IPLC image with H&E staining (×400). IPLC cells had enlarged nuclei and abundant eosinophilic granular C cytoplasm. |
 | Figure 2Univariate analysis curves for relapse-free survival (RFS) and disease-specific survival (DSS) of patients with invasive ductal carcinoma (IDC) and invasive pleomorphic lobular carcinoma (IPLC). (A) Univariate analysis curves for RFS of IDC and IPLC. (B) Univariate analysis curves for DSS of IDC and IPLC. |
 | Figure 3Multivariate analysis curves for relapse-free survival (RFS) and disease-specific survival (DSS) of patients with invasive ductal carcinoma (IDC) and invasive pleomorphic lobular carcinoma (IPLC). (A) Multivariate analysis curves for RFS of IDC and IPLC. (B) Multivariate analysis curves for DSS of IDC and IPLC. |
References
1. Page DL, Anderson TJ. Diagnostic Histopathology of the Breast. 1997. Edinburgh: Churchill Livingstone.
2. Eusebi V, Magalhaes F, Azzopardi JG. Pleomorphic lobular carcinoma of the breast: an aggressive tumor showing apocrine differentiation. Hum Pathol. 1992. 23:655–662.

3. Weidner N, Semple JP. Pleomorphic variant of invasive lobular carcinoma of the breast. Hum Pathol. 1992. 23:1167–1171.

4. Yoder BJ, Wilkinson EJ, Massoll NA. Molecular and morphologic distinctions between infiltrating ductal and lobular carcinoma of the breast. Breast J. 2007. 13:172–179.

5. Vargas AC, Lakhani SR, Simpson PT. Pleomorphic lobular carcinoma of the breast: molecular pathology and clinical impact. Future Oncol. 2009. 5:233–243.

6. Jacobs M, Fan F, Tawfik O. Clinicopathologic and biomarker analysis of invasive pleomorphic lobular carcinoma as compared with invasive classic lobular carcinoma: an experience in our institution and review of the literature. Ann Diagn Pathol. 2012. 16:185–189.

7. Buchanan CL, Flynn LW, Murray MP, Darvishian F, Cranor ML, Fey JV, et al. Is pleomorphic lobular carcinoma really a distinct clinical entity? J Surg Oncol. 2008. 98:314–317.

8. Bentz JS, Yassa N, Clayton F. Pleomorphic lobular carcinoma of the breast: clinicopathologic features of 12 cases. Mod Pathol. 1998. 11:814–822.
9. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 2010. 7th ed. New York: Springer.
10. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998. 11:155–168.
11. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999. 17:1474–1481.

12. Varga Z, Zhao J, Ohlschlegel C, Odermatt B, Heitz PU. Preferential HER-2/neu overexpression and/or amplification in aggressive histological subtypes of invasive breast cancer. Histopathology. 2004. 44:332–338.

13. Gennari R, Curigliano G, Rotmensz N, Robertson C, Colleoni M, Zurrida S, et al. Breast carcinoma in elderly women: features of disease presentation, choice of local and systemic treatments compared with younger postmenopasual patients. Cancer. 2004. 101:1302–1310.
14. Herbsman H, Feldman J, Seldera J, Gardner B, Alfonso AE. Survival following breast cancer surgery in the elderly. Cancer. 1981. 47:2358–2363.

15. Holli K, Isola J. Effect of age on the survival of breast cancer patients. Eur J Cancer. 1997. 33:425–428.

16. Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011. 43:1–11.

17. Orvieto E, Maiorano E, Bottiglieri L, Maisonneuve P, Rotmensz N, Galimberti V, et al. Clinicopathologic characteristics of invasive lobular carcinoma of the breast: results of an analysis of 530 cases from a single institution. Cancer. 2008. 113:1511–1520.

18. Rakha EA, El-Sayed ME, Menon S, Green AR, Lee AH, Ellis IO. Histologic grading is an independent prognostic factor in invasive lobular carcinoma of the breast. Breast Cancer Res Treat. 2008. 111:121–127.

19. Bane AL, Tjan S, Parkes RK, Andrulis I, O'Malley FP. Invasive lobular carcinoma: to grade or not to grade. Mod Pathol. 2005. 18:621–628.

20. Middleton LP, Palacios DM, Bryant BR, Krebs P, Otis CN, Merino MJ. Pleomorphic lobular carcinoma: morphology, immunohistochemistry, and molecular analysis. Am J Surg Pathol. 2000. 24:1650–1656.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download