Abstract
We describe the magnetic resonance imaging (MRI) findings of 13 cm-sized low-grade angiosarcoma of the breast that occurred in a 23-year-old woman. Magnetic resonance examination revealed an ill-defined mass with marked high-signal intensity on T2-weighted images and persistent heterogeneous enhancement. Thirty months later she developed bone metastases, incidentally found on an MRI performed to evaluate the pelvis. There were well-defined bone lesions with high-signal intensity on T2-weighted images and persistent contrast enhancement on delayed phases. The metastases were not detected on previous computed tomography and fluoro-deoxyglucose positron emission tomography scans because the lesions were subtle osteoblastic type with a low proliferative index.
Angiosarcoma of the breast is a rare tumor with a poor prognosis [1]. A disease-free survival depends on the tumor grade [2,3] and size [4]. We report a case of a young woman treated for large low-grade angiosarcoma of the breast who developed bone metastases 30 months after primary treatment. Magnetic resonance imaging (MRI) detected wide spread metastases to the pelvis and lower lumbar spine, which were false negatives on computed tomography (CT) and fluoro-deoxy-glucose positron emission tomography (FDG-PET) scans. Follow-up MRI may play an important role in the early detection of bone metastases in the case of patients treated for large low-grade angiosarcoma.
The patient is a 23-year-old woman with spastic paraplegia since infancy that presented with increasing size and discomfort of the right breast in June 2007.
A physical examination found that the right breast was diffusely swollen but soft. No discrete mass was found and there were no associated signs of inflammation. Sonography showed diffuse abnormal mixed hyper- and hypoechogenicity of breast tissue without a discrete mass.
MRI examination was performed with a 1.5 Tesla unit (Signa Excite HD; GE Healthcare, Milwaukee, USA) using a dedicated breast coil (GE 4 channel breast array coil) and it revealed an ill-defined large mass that occupied the outer quadrants and lower inner quadrant of the right breast with marked high-signal intensity on T2-weighted images (Figure 1A) and low-signal intensity on T1-weighted images. The lesion diameters measured in the axial plane were 11.5×8.5 cm and the longitudinal diameter was 8 cm. There were also skin thickening and edema, prepectoral fluid and ipsilateral increased breast vascularity. Dynamic sagittal 3D-Vibrant MR images obtained after administration of gadolinium-chelate showed persistent heterogeneous enhancement of the mass (Figure 1B and 1C) with time signal intensity curves type 1A and 1B. Initial peak intensity enhancement values ranged from 50% to 140%. No enlarged axillary lymph nodes were identified.
After an incisional biopsy, a right mastectomy with axillary lymph nodes biopsy was performed. Pathologic examination showed 13 cm-sized low-grade angiosarcoma with positive margins; the skin and lymph nodes were free of the tumor. In consideration of the low-grade tumor and positive margins, radiotherapy without chemotherapy was recommended.
Thirty months after breast surgery, follow-up chest, abdominal and pelvis CT scans (Figure 1D) showed a suspicious retroperitoneal right pelvic lymph node (1.5 cm diameter) with a low metabolic activity on FDG-PET (SUVmax 1.15). Therefore, the patient underwent pelvic MRI, which confirmed the presence of a retroperitoneal lymph node (Figure 1E) and revealed multiple well-defined bone lesions in the pelvis and lower spine, with high-signal intensity on T2-weighted images (Figure 1F) and low-signal intensity on T1-weighted images. These lesions presented persistent contrast enhancement on more delayed phases, suggestive of metastases (Figure 1G).
The patient underwent a CT-guided biopsy of the right ilium that confirmed the presence of metastatic angiosarcoma with a low proliferative index (Ki-67 lower than 1%), and that lesion was negative on CT and PDF-PET scans. Palliative radiotherapy was recommended for larger bone lesions of the pelvis. However, follow-up MRI over the course of 2 years showed progression of disease.
Primary angiosarcoma of the breast is a rare type of tumor that accounts for 0.04% of malignant breast neoplasm and arises in younger women, usually between the ages of 30 to 40 [5,6]. Several studies have demonstrated that clinical signs are usually non-specific and that MRI can detect and characterize the lesion better than mammography and ultrasonography [1,7].
In this study we described a huge mass of markedly high-intensity on T2-weighted images and with prolonged enhancement after intravenous contrast administration, according to previous reports [2,7]. This dynamic behaviour is likely correlated to histological features including blood-filled vascular spaces and vascular channels.
This type of tumor also has a poor prognosis and tends to spread through the blood stream rather than through the lymph node system [1,8].
The prognosis depends on the histological grade and tumor size. Patients with high-grade tumor tend to have lower survival rates and higher recurrence rates [2].
Sher et al. [9] reported recurrent disease in 55% of the patients with primary angiosarcoma after a median follow-up of only 40 months and the most common site of recurrence was locoregional location (52.6%) followed by liver (13.2%), bone (10.5%), and lung (10.5%). Vorburger et al. [4] described recurrent disease in 28% of patients with primary angiosarcoma of the breast: 4 had locoregional recurrences and 5 distant metastatic disease. The median time to the first recurrence was 3.7 years. In these studies, the disease-free survival was significantly associated with the primary tumor size on multivariate analysis and not with the histological grade; in fact, the patients affected by angiosarcoma with size of more than 5 cm were associated with lower disease-free survival.
Our young patient presented bone metastases 30 months after diagnosis of primary angiosarcoma measuring 13 cm.
In our report MRI was particularly useful for identifying osteoblastic bone metastases, that were not detected on CT scan because they consisted of patchy areas of subtle ground glass increased attenuation.
Osteoblastic proliferation results in an increase in the bone matrix with a relative decrease in cell density and the SUV could be low on FDG-PET [10].
The SUV on FDG-PET is also correlated to the degree of malignancy [10]. Gatcombe et al. [11] described a case of a patient with high-grade angiosarcoma of the breast who developed bone metastases detected with PET scan. In our report FDG-PET did not identify bone metastases because they were of the osteoblastic type with a low proliferative index.
In conclusion, in the case of young woman with increasing size of the breast, MRI could play an important role to identify and characterize breast lesion. MRI should also be considered as a useful diagnostic tool for the detection of bone metastases in patients previously operated for low-grade angiosarcoma larger than 5 cm.
Figures and Tables
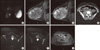 | Figure 1A low-grade angiosarcoma in a 23-year-old woman with increasing size and discomfort of the right breast. (A) Axial T2-weighted fast spin-echo magnetic resonance image shows a marked high-signal intensity mass of the right breast, mainly in the outer quadrants. (B, C) Dynamic sagittal 3D Vibrant early- (B) and late- (C) phase images show persistent heterogeneous enhancement of the mass. (D) Post-contrast computed tomography image shows suspicious right retroperitoneal pelvic lymph node (arrow). (E, F) Axial T2-weighted fast spin-echo fat saturation images confirmed retroperitoneal lymph node (E, arrow) and revealed well-defined high-signal intensity bone lesions (F). (G) Axial 3D-Liver Acquisition with Volume Acceleration images obtained late after intravenous contrast administration. The bone lesions show persistent contrast enhancement on delayed phases, suggestive of metastases. |
References
1. Kikawa Y, Konishi Y, Nakamoto Y, Harada T, Takeo M, Ogata M, et al. Angiosarcoma of the breast: specific findings of MRI. Breast Cancer. 2006. 13:369–373.

2. Lim RF, Goei R. Best cases from the AFIP: angiosarcoma of the breast. Radiographics. 2007. 27:Suppl 1. S125–S130.
3. Monroe AT, Feigenberg SJ, Mendenhall NP. Angiosarcoma after breast-conserving therapy. Cancer. 2003. 97:1832–1840.

4. Vorburger SA, Xing Y, Hunt KK, Lakin GE, Benjamin RS, Feig BW, et al. Angiosarcoma of the breast. Cancer. 2005. 104:2682–2688.

5. Yang WT, Hennessy BT, Dryden MJ, Valero V, Hunt KK, Krishnamurthy S. Mammary angiosarcomas: imaging findings in 24 patients. Radiology. 2007. 242:725–734.

6. Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. AJR Am J Roentgenol. 2008. 190:533–538.

7. Marchant LK, Orel SG, Perez-Jaffe LA, Reynolds C, Schnall MD. Bilateral angiosarcoma of the breast on MR imaging. AJR Am J Roentgenol. 1997. 169:1009–1010.

8. Murakam S, Nagano H, Okubo K, Sakata H, Tsuji Y, Ishiguro T, et al. Angiosarcoma of the breast: report of a case and its findings of MRI. Breast Cancer. 2001. 8:254–258.

9. Sher T, Hennessy BT, Valero V, Broglio K, Woodward WA, Trent J, et al. Primary angiosarcomas of the breast. Cancer. 2007. 110:173–178.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download