Abstract
Synchronous bilateral breast cancer is extremely rare in men and has not, up to date, been reported in Korea. A 54-year-old man presented with a palpable mass in the right breast. The right nipple was retracted and bilateral axillary accessory breasts and nipples were present. On physical examination, a 2 cm-sized mass was palpated directly under the right nipple, and, with squeezing, bloody discharge developed in a single duct of the left nipple. There was no palpable mass in the left breast, and axillary lymph nodes were not palpable. Physical examination of external genitalia revealed a unilateral undescended testis on the left side. Synchronous bilateral breast cancer was diagnosed using mammography, ultrasonography, and core-needle biopsy. Histopathological examination revealed invasive ductal carcinoma in the right breast and ductal carcinoma in situ in the left breast. Bilateral total mastectomy, sentinel lymph node biopsy, and excision of accessory breasts in the axilla were performed.
Male breast cancer is a rare condition, accounting for <1% of all breast cancers [1,2]. Moreover, synchronous bilateral male breast cancer is an extremely rare disease which has not been previously reported in Korea. Synchronous bilateral involvement is estimated to be more uncommon than metachronous bilateral involvement, and the former accounts for approximately one-third of all bilateral breast cancers [2].
Most cases of male breast cancer are detected between the ages of 60 and 70, and the mean age is 67 years, which is older than that the mean age for women [3,4]. Because male breast cancer tends to be diagnosed at an older age, and at a more advanced stage than female breast cancer, overall survival rate is lower for men [5]. However, outcomes are comparable when age at diagnosis and stage are adjusted [4].
Several risk factors, including familial and genetic factors (BRCA2), radiation exposure, Klinefelter's syndrome (47, XXY), hormonal imbalance, obesity and testicular disease (undescended testis, orchitis, orchectomy) have been proposed [1]. We report here a case of synchronous bilateral breast cancer in a man who had an undescended testis on the left side.
A 54-year-old man presented with a palpable mass in the right breast. There was no history of familial breast cancer, gynecomastia, solid organ tumors or hormonal medication. He was not a heavy drinker and had a smoking history of 20 pack years. Body mass index (BMI) was 24.5 (height, 154 cm; body weight, 58 kg). The right nipple was retracted and bilateral axillary accessory breasts with nipples were present (Figure 1).
On physical examination, a 2 cm-sized mass was palpated just under the right nipple and bloody discharge developed, with squeezing, from a single duct of the left nipple. There was no palpable mass in the left breast, and axillary lymph nodes were not palpable. Physical examination of the external genitalia revealed unilateral undescended testis, which was small (7-8 mL) and located in the left inguinal canal. The size of the right testis was normal (15 mL).
The patient's hormonal profiles, including testosterone, prolactin and thyroid function tests, were normal. However, levels of estradiol 58.9 pg/mL (normal range [NR], 15-47), luteinizing hormone (LH) 6.3 m/U/mL (NR, 1.0-5.3), and follicular stimulating hormone (FSH) 10.9 m/U/mL (NR, 1.0-5.3) were slightly increased. Chromosomal studies revealed a normal male karyotype of 46XY, and genetic analyses for BRCA1/2 genes were normal. Radiologic examination, including chest radiograph, liver ultrasonography, and F-18-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT), showed no evidence of metastatic disease. Mammography and ultrasonography showed a mass (BIRADS Category 5) in the subareolar area of both breasts (Figures 2, 3). In preoperative PET/CT, bilateral uptake in the subareolar area of SUVmax 6.8 in the right breast and 5.2 in the left breast (Figure 4), and mild uptake in both axillaes were shown. Core-needle biopsy revealed invasive ductal carcinoma in the right breast and ductal carcinoma in situ in the left breast. Fine needle aspiration cytology of the right axillary lymph node was negative.
Bilateral total mastectomy, sentinel lymph node biopsy, and excision of the accessory breasts in the axilla were performed. Histopathological examination revealed invasive ductal carcinoma in the right breast (maximum diameter, 2.2 cm), and solid papillary carcinoma, a variant of ductal carcinoma in situ in the left breast. The invasive ductal carcinoma in the right breast was composed primarily of micropapillary components, and the tumor cells were suspended in a clear space. The solid papillary carcinoma in the left breast showed solid tumor cell nests with focal necrosis (Figure 5). TNM staging was pT2N0 (sn) in the right breast and pTisN0 (sn) in the left breast. There was no evidence of tumor invasion to the pectoralis major muscle in the right breast. Immunohistochemical staining of the right breast cancer showed estrogen receptor (ER) 3+/3 (>95%), progesterone receptor (PR) 2+/3 (40%) and c-erbB-2 1+/3 (40%). Adjuvant chemotherapy and hormonal therapy were planned.
Bilateral male breast cancer is very rare, and the incidence is reported to be only 1.5% to 2% of all male breast cancers. The incidence of metachronous breast cancer is higher than that of synchronous breast cancer [6,7]. Literature reviews on synchronous bilateral male breast cancer, using PubMed until August 2011, were performed, and 24 cases were searched [3,6]. Sporadic single case reports of synchronous bilateral male breast cancer, associated with male potential hypogonadism, hyperprolactinemia, hormonal therapy due to prostate cancer, long standing gynecomastia, and chromosomal abnormality (XXY or 45,X/46,XY mosaic karyotype), have been reported [8-10].
The most common presenting symptoms of male breast cancer are a painless lump, bloody nipple discharge, and nipple retraction. The most common sign of breast cancer in men is a firm, non-tender mass located in the subareolar area. Nipple discharge is more common in malignant rather than benign disease in men [4,11].
Various risk factors for male breast cancer have been identified, including familial and genetic factors (BRCA2), radiation exposure, Klinefelter's syndrome (47, XXY), hormonal imbalance, obesity, and testicular disease (undescended testis, orchitis, orchectomy) [1,11]. About 4% to 16% of male breast cancers are reported to be associated with BRCA2 mutation in population-based series [4]. However, the role of BRCA1 mutation has not been clearly defined with regard to male breast cancer. Chromosomal studies revealed a normal male karyotype of 46XY, and genetic analyses for BRCA1/2 genes were normal in the case of our subject.
Undescended testis has been investigated as a risk factor for male breast cancer. According to one report, there is a 12-fold increased risk of breast cancer in patients with undescended testis [8]. This may be the result of a hormonal imbalance caused by testicular dysfunction [12]. Unilateral undescended testis usually does not induce abnormal level of LH and FSH due to compensatory growth of the remaining gonad [13]. The elevated gonadotropin level in this case may have been due to hypogonadism caused by testicular dysfunction. Other testicular disorders, including mump orchitis, orchiectomy and testicular injury, have also been mentioned as risk factors for male breast cancer [12].
Obesity in postmenopausal women and men has been considered a risk factor for breast cancer, due to conversion of testosterone to estradiol and androstenedione to estrone in peripheral adipose tissue. Several studies indicate that mean estradiol levels were higher in male breast cancer patients than in controls [1]. The slightly elevated level of estrogen in this case might be attributed to the patient's mildly increased BMI. However, we cannot conclude that bilateral breast cancer developed due to this hormonal imbalance.
Accessory breasts, or polymastia, occur in 0.4% to 6% of females and 1% to 3% of males [14]. A case of bilateral accessory breasts in the axilla, with nipples, in a male patient with bilateral synchronous breast cancer has not been described before. The clinical significance of accessory breasts in male breast cancer is unknown.
Male breast cancer is more prone to test positive for hormonal receptors, but less likely to show c-erbB-2 over-expression [15]. Of the 24 cases of bilateral synchronous male breast cancer reported in the literature, 20 cases, including our case, were analyzed for hormonal receptors and c-erbB-2 status. The hormonal receptor (ER or PR) was positive in 14 out of 16 cases (87.5%), while c-erbB-2 was negative in all of the 7 cases that were analyzed [3,6]. Unilateral and bilateral breast cancer in men showed similar hormonal status [4]. There is no data comparing the outcomes of male breast cancer with regard to differences between unilateral and bilateral cases.
In conclusion, the rarity of bilateral breast cancer in men often makes us overlook the importance of examination of the contralateral breast. We should take into account the possibility of synchronous breast cancer in male breast cancer patients, especially in patients with an undescended testis and elevated estrogen levels. A thorough physical examination is recommended in such cases.
Figures and Tables
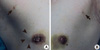 | Figure 1A mass (arrowheads) with nipple retraction was detected in the right breast. Bilateral accessory breasts in the axilla with nipples (arrows) were also present. (A) Right. (B) Left. |
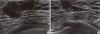 | Figure 3Ultrasonography showed a hypoechoic breast mass (BIRADS Category 5) in the subareolar area of both breasts. (A) Right. (B) Left. |
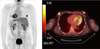 | Figure 4Positron emission tomography/computed tomography showed bilateral uptake in the subareolar area. SUVmax was 6.8 in the right breast and 5.2 in the left breast. |
 | Figure 5(A) The invasive ductal carcinoma in the right breast was mainly composed of micropapillary components (H&E stain, ×200). The tumor cells were suspended in a clear space. (B) The solid papillary carcinoma in the left breast showed solid tumor cell nests with focal necrosis (H&E stain, ×100). |
References
1. Weiss JR, Moysich KB, Swede H. Epidemiology of male breast cancer. Cancer Epidemiol Biomarkers Prev. 2005. 14:20–26.
2. Coard K, McCartney T. Bilateral synchronous carcinoma of the male breast in a patient receiving estrogen therapy for carcinoma of the prostate: cause or coincidence? South Med J. 2004. 97:308–310.

3. Yaman E, Ozturk B, Coskun U, Buyukberber S, Kaya AO, Yildiz R, et al. Synchronous bilateral breast cancer in an aged male patient. Onkologie. 2010. 33:255–258.

4. Giordano SH. A review of the diagnosis and management of male breast cancer. Oncologist. 2005. 10:471–479.

5. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004. 101:51–57.
6. Lambley J, Maguire E, Yin Lam K. Synchronous bilateral breast cancer in an elderly man. Breast J. 2005. 11:153.

7. Cutuli B, Lacroze M, Dilhuydy JM, Velten M, De Lafontan B, Marchal C, et al. Male breast cancer: results of the treatments and prognostic factors in 397 cases. Eur J Cancer. 1995. 31A:1960–1964.

8. Hoque HM, Kothari A, Hamed H, Fentiman IS. Synchronous bilateral breast cancer in a patient with Klinefelter's syndrome. Int J Gen Med. 2010. 3:19–21.
9. Hirose Y, Sasa M, Bando Y, Hirose T, Morimoto T, Kurokawa Y, et al. Bilateral male breast cancer with male potential hypogonadism. World J Surg Oncol. 2007. 5:60.

10. Qureshi K, Athwal R, Cropp G, Basit A, Adjogatse J, Bhogal RH. Bilateral synchronous ductal carcinoma in situ in a young man: case report and review of the literature. Clin Breast Cancer. 2007. 7:710–712.

11. Leinung S, Horn LC, Backe J. Male breast cancer: history, epidemiology, genetic and histopathology. Zentralbl Chir. 2007. 132:379–385.
12. Thomas DB, Jimenez LM, McTiernan A, Rosenblatt K, Stalsberg H, Stemhagen A, et al. Breast cancer in men: risk factors with hormonal implications. Am J Epidemiol. 1992. 135:734–748.

13. Gómez-Pérez R, Osuna JA, Arata-Bellabarba G. Surgical vs. untreated cryptorchidism: effects on fertility. Arch Androl. 2004. 50:19–22.

14. Lesavoy MA, Gomez-Garcia A, Nejdl R, Yospur G, Syiau TJ, Chang P. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995. 35:356–360.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download