Abstract
Metastasis to the breast from extra-mammary tumors is rare with only a few sporadic cases reported. We present a 58-year-old female patient diagnosed with renal cell carcinoma. Five years ago she had a radical nephrectomy and was free of disease, then discovered solitary breast mass following self-examination. The patient presented to the breast clinic for evaluation whereupon the breast mass was identified on physical and radiological examinations. Fine needle aspiration was diagnostic of metastatic renal cell carcinoma and subsequent imaging studies demonstrated multiple pulmonary deposits and recurrent renal mass in the tumor bed of the diseased site. In a multidisciplinary clinic, the patient was elected for excision biopsy followed by systemic tyrosine kinase inhibitor therapy. Six months later she had brain metastasis and received whole brain irradiation followed by palliative therapy. We are presenting this rare case with the aim of increasing awareness of breast secondaries.
Renal cell carcinoma is one of the most aggressive urological tumors and account for 3% of all neoplasms in adults. It is the third most common genitourinary tract tumor [1]. Thirty percent of patients with renal cell carcinoma have metastasis at the time of diagnosis, most commonly in the lung (70%), lymph nodes (55%), bone (42%), liver (41%), adrenal gland (15%), and central nervous system (11%) [1,2]. Breast involvement with malignant tumor is extremely rare. The incidence of breast metastasis from extra-mammary primary tumors ranges from 0.5% to 2% [3,4]. The primary tumors most commonly causing breast metastasis include melanoma, lymphoma, and leukemia [5]. Metastases from renal carcinoma in the breast are exceptional, and only 14 cases have been reported. Metastasis was the first sign of disease in seven of these cases [5]. However, breast secondaries mimic breast cancer in clinical examination. Diagnosis of an extramammary metastasis is crucial to avoid unnecessary mastectomy and begin treatment for the primary tumor. Awareness of breast secondaries and meticulous diagnosis are much needed. We report a rare case of primary renal carcinoma metastasis to the breast after 5 years and 7 months after the patient had left radical nephrectomy. This is the first case to be reported from the Arab Middle Eastern region.
A 58-year-old woman was admitted to the hospital on 15 August, 2003 with severe left loin pain, and hematuria. Abdominal ultrasonography revealed left loin mass. Subsequent computed tomography (CT) scan confirmed a left renal mass of 6×7 cm confined to the left kidney with no evidence of spread or vascular invasion. The patient underwent left radical nepherectomy on 25 August, 2003. Pathological examination demonstrated renal cell carcinoma, clear cell type grade II. The tumor was limited to the kidney with no invasion of perinephric tissue or vascular invasion. Surgical margins were free and the patient was classified as tumor stage II (T2aN0M0). The patient was followed up every 3 months for 2 years then annually for 3 years guided by clinical examination, annual laboratory investigations and radiological imaging. Her last CT scan evaluation was September 2008, and was unremarkable with no detected disease compared with previous scans. Her annual visit was 4 months away when she discovered her breast lesion. She was disease free clinically and radiologically until March 2009, when the patient presented to the breast clinic with a painless, palpable, and rapidly growing left breast mass discovered during breast self examination 2 months previously. Clinical examination confirmed a non-tender, palpable, mobile, and firm mass in the upper outer quadrant of left breast at the 3 o'clock position with no skin infiltration. There was no clinically palpable axillary lymphadenopathy.
Bilateral mammography showed a dense well circumscribed solid mass, speculated intramammary lesion measuring 4×3 cm located in the lower outer quadrant of the left breast with adjacent parenchymal distortion and no signs of micro or macrocalcifications, and no skin or nipple retraction (Breast Imaging Reporting and Data System [BIRADS] score was IV of left breast) (Figure 1). No evidence of enlarged lymph nodes. For the right breast, the BIRAD score was I, and no intramammary lesion. Breast ultrasonography showed a large well defined hypoechoic heterogeneous solid mass intramammary lesion with internal solid components that showed some vascularity. The adjacent parenchyma was just displaced without obvious distortion with no skin thickening or nipple retraction or duct ectasia. No evidence of enlarged lymph nodes. The right breast was unremarkable.
Fine needle aspiration biopsy (FNAB) was carried out and the morphological diagnosis was malignant epithelial neoplasm, consistent with renal clear cell carcinoma, metastatic deposits (Figure 2). Tumor cells were strongly positive for vimentin.
The patient denied any chest, abdominal, urinary or neurological symptoms and her clinical abdominal examination was unremarkable. A whole body CT scan was carried out and CT of the chest showed a left breast mass with central nonenhanced hypodensity and surrounded by fat stranding and skin density (Figure 3). Multiple pulmonary deposits of variable sizes were identified with most of the nodules subpleural in location (Figures 3, 4). Abdominal CT scans revealed a left renal mass in the left renal bed which showed necrotic nonenhanced area with involvement of the left psoas muscle (Figure 5). There was thrombus noted in inferior vena cava. A whole body bone scan with 99mTc was negative for bone metastatic spread. The patient's hemoglobin was 9.1 g/dL, her calcium level was 9.2 mg/dL, and LDH was 720 IU/L (patient's predictors of short survival score was 3). Her case was discussed in a multidisciplinary meeting and, the breast lump was excised on 26 April 2009. Pathological examination showed metastatic clear cell renal carcinoma, with free surgical margins. The patient was referred to the renal oncologist for further management. Her performance status was II ECOG, and she was asymptomatic regarding local recurrence and pulmonary metastatic deposits. She received systemic therapy oral tyrosine kinase inhibitor sunitinib with good tolerance and a stationary course.
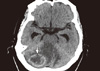
Six months after breast lump excision, the patient presented to the emergency room (ER) with repeated vomiting, and headache. CT of the brain with contrast revealed two metastatic deposits, the largest measuring 4×3×1.5 cm in the cerebellar region (Figure 6). Craniotomy and excision biopsy was carried out and the morphological pattern demonstrated, metastatic deposit of renal clear cell carcinoma (Figure 7).
The patient received whole brain irradiation 30 Gy/10 fractions/2 weeks, and she tolerated her treatment very well. Two months later she developed progressive intolerable left renal pain with radiological disease progression. The pulmonary deposits and left renal mass had both increased in size. Sunitinib was stopped and she was not a candidate for second line active therapy because of her performance status (PS III ECOG). The patient referred to the palliative care unit for palliative therapy. She survived for 81 months after diagnosis of her primary disease and 14 months following the development of breast secondaries.
The breast is a rare site for metastatic disease. Metastatic neoplasm to the breast accounts for 0.5% to 6.6% of all malignant tumors in autopsy series [6]. Reported primary malignant tumors that spread to the breast include melanoma [7], kidney [8], stomach [8], uterine, vulva [9], and lung cancer [10]. It is predominantly a disease of women, similar to that observed in primary breast cancers. There is minimal information in the literature regarding the prognosis of patients with metastatic disease to the breast; most information of this topic consists of case reports and small series [11]. Renal cell carcinoma is unfavorable for its hematogenous spread, and metastasis to the breast is extremely uncommon [6], however, in our case the breast metastasis was the first presentation of disease recurrence after 67 months of the primary disease. Clinically, metastatic lesions in the breast present as painless masses with rapid growth, the skin is not usually affected and axillary lymph node involvement is uncommon [4,7,12,13]. A mammogram may show solid tumor with lack of micro-calcification. Solitary lesion may occur in 85%, and bilateral affection of both breasts is not uncommon [4,11]. Differentiation of primary breast cancer from secondary metastasis is very important; as such features may be helpful. A history of malignant disease should arouse suspicion of disease spread, although, primary breast cancer is a common disease. Triple assessment should be performed on the breast lump (FNAB, core or excision biopsy, and immunohistochemistry), for accurate diagnosis [14,15]. Excision biopsy is a good treatment option and reported by most series as the best disease control [5,11,15]. In fact, the appropriate treatment in these cases remains controversial [7,11]. Mastectomy and lymph node dissection are unnecessary, cure is unlikely with metastatic renal cancer and palliative radiotherapy and target therapy are considered to be good local and systemic control with tolerable toxicity [7,11,15]. In our case, the patient had scored 3 in predictors of short survival and survived for 14 months from the time of disease metastasis and it is comparable with the other few sporadic series [7]. She had three sites of metastasis (breast, pulmonary, and brain) besides the local recurrence. There are no effective therapeutic options for disseminated renal cell carcinoma. The inability to predict either an individual patient's longevity or whether a patient will develop local complication of metastatic disease makes treatment decision-making difficult. There is minimal information in the literature regarding the prognosis of patients with metastatic disease to the breast and further studies are warranted.
In conclusion, primary and secondary breast cancer may mimic each other clinically and radiologically. Careful assessment and adequate tissue examination are mandatory for confirmation of the diagnosis. Resection of isolated metastasis from renal cell carcinoma is mostly feasible and can achieve long term disease control.
Figures and Tables
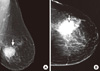 | Figure 1Imaging finding mediolateral (A) and craniocaudal (B) views of a mammogram which showed left breast well circumscribed high-density mass (arrows) with no microclassifications. |
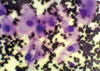 | Figure 2Fine needle aspiration from left breast mass which was showing tumor cells with atypical nuclei and abundant cytoplasm with vacuolation and granularity (Diff-Quik stain, ×400). |
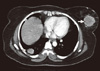 | Figure 3Contrast enhanced axial computed tomography image of the chest (mediastinal window) showed heterogeneously enhanced left breast mass (arrows) and solitary right peripherally located pulmonary. |
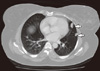 | Figure 4Contrast enhanced axial computed tomography image of the chest (lung window) showed tow peripherally located left pulmonary deposits (arrows). |
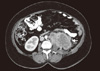 | Figure 5Contrast enhanced axial computed tomography image of the abdomen showed heterogeneously enhanced mass (tumor) in the left renal bed (arrow) with invasion to left psoas muscle. |
References
1. Wahner-Roedler DL, Sebo TJ. Renal cell carcinoma: diagnosis based on metastatic manifestations. Mayo Clin Proc. 1997. 72:935–941.

2. Tarraza HM Jr, Meltzer SE, DeCain M, Jones MA. Vaginal metastases from renal cell carcinoma: report of four cases and review of the literature. Eur J Gynaecol Oncol. 1998. 19:14–18.
4. Vassalli L, Ferrari VD, Simoncini E, Rangoni G, Montini E, Marpicati P, et al. Solitary breast metastases from a renal cell carcinoma. Breast Cancer Res Treat. 2001. 68:29–31.

5. McLauglin SA, Thiel DD, Smith SL, Wehle MJ, Menke DM. Solitary breast mass as initial presentation of clinically silent metastatic renal cell carcinoma. Breast. 2006. 15:427–429.

6. Pagano S, Franzoso F, Ruggeri P. Renal cell carcinoma metastases. Review of unusual clinical metastases, metastatic modes and patterns and comparison between clinical and autopsy metastatic series. Scand J Urol Nephrol. 1996. 30:165–172.
7. Vaughan A, Dietz JR, Moley JF, Debenedetti MK, Aft RL, Gillanders WE, et al. Metastatic disease to the breast: the Washington University experience. World J Surg Oncol. 2007. 5:74.

8. Gacci M, Orzalesi L, Distante V, Nesi G, Vezzosi V, Livi L, et al. Renal cell carcinoma metastatic to the breast and breast cancer metastatic to the kidney: two rare solitary metastases. Breast J. 2005. 11:351–352.

9. Hsiao HH, Liu YC, Hou MF, Lin SF. Uterine leiomyosarcoma metastasis to the breast. Eur J Gynaecol Oncol. 2008. 29:191–192.
10. Sawada T, Takahashi H, Hasatani K, Yoshida I, Oyama O, Inoue R, et al. Tumor-to-tumor metastasis: report of an autopsy case of lung adenocarcinoma metastasizing to renal cell carcinoma. Intern Med. 2009. 48:1525–1529.

11. Alzaraa A, Vodovnik A, Montgomery H, Saeed M, Sharma N. Breast metastasis from a renal cell cancer. World J Surg Oncol. 2007. 5:25.

12. Vergier B, Trojani M, de Mascarel I, Coindre JM, Le Treut A. Metastases to the breast: differential diagnosis from primary breast carcinoma. J Surg Oncol. 1991. 48:112–116.

13. Hardy SC, Benson EA. Solitary breast metastasis from a hypernephroma. Eur J Surg Oncol. 1987. 13:365–366.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download