Abstract
Purpose
Little information exists about the possible influence of serum HER2/neu on response to chemotherapy. We propose that the assessment of serum HER2/neu in a pretreatment serum sample may be useful in predicting response to neoadjuvant chemotherapy.
Methods
All breast cancer patients were tested by immunohistochemical stain and fluorescent in situ hybridization for HER2/neu before treatment. Serum HER2/neu was twice measured by chemiluminescence immunoassay (ADVIA Centaur System) before neoadjuvant chemotherapy and before operation. The cut-off value was 10.2 mg/mL, according to the previous study. Pathologic complete response (pCR) was considered as no residual tumor or remnant ductal carcinoma in situ; partial response (PR) was a less than 50% decrease in maximal diameter in pathologic tumor size. The measurements for the changes of serum HER2/neu were defined as pretreatment HER2/neu-preoperation HER2/neu. We compared the change of serum HER2/neu between that from before chemotherapy and that after chemotherapy, the pathologic complete response and partial response, and the trastuzumab group and anthracycline group.
Results
Serum HER2/neu was decreased after neoadjuvant chemotherapy. The mean of serum HER2/neu in prechemotherapy was 15.4±9.0 ng/mL, and that of postchemotherapy was 10.5±2.0 ng/mL (p=0.04). Pathologic response was correlated with the change of serum HER2/neu (PR, 11.7±2.2 ng/mL vs. pCR, 23.7±13.1 ng/mL; p=0.01). In the trastuzumab group, pCR was marginally correlated with the change of serum HER2/neu (PR, 0.8±0.84 ng/mL vs. pCR, 21.1±13.2 ng/mL; p=0.08).
Conclusion
Serum HER2/neu levels during treatment were associated with pathologic response in patients receiving neoadjuvant chemotherapy, particularly, in a trastuzumab-based regimen. The change of serum HER2/neu levels may serve in monitoring neoadjuvant therapy in HER2/neu-overexpressed breast cancer.
The proto-oncogene in human breast cancer, c-erbB-2, is also known as HER2/neu. It is located on chromosome 17q21 and encodes a 185 kDa glycoprotein that shares homology with the epidermal growth factor receptor [1]. As a result of gene amplification, 20% to 30% of breast cancer shows overexpressed-HER2 protein, and such overexpression is associated with poor prognosis [2,3] The enzyme-linked immunosorbent assay (ELISA) method for measuring the circulating HER2/neu extracellular domain (ECD) is to obtain the real-time status of HER2/neu and has been evaluated to monitor changes in the HER2/neu ECD concentrations after surgery. After proteolytic processes, the ECD portion is shed in to the blood stream [4]. Thus, the shed ECD has been shown to be present in the sera of healthy women and to be elevated to the normal range in women with breast cancer and, in particular, women with metastatic breast cancer. Elevated HER2/neu ECD concentrations are observed in 3% to 12% of primary breast cancer [5,6]. Recent studies have demonstrated that increased HER2/neu ECD serum levels are associated with a poor prognosis and may also indicate therapy resistance [7,8]. Several studies on endocrine treatment with tamoxifen revealed an impaired therapy effect in patients with HER2/neu overexpression in breast carcinoma tissue or with elevated serum levels of HER2/neu ECD [9,10]. Two smaller published reports investigated the correlation between pretreatment serum HER2/neu and pathologic complete response during neoadjuvant chemotherapy plus trastuzumab. Kosteler et al. [11] evaluated serum HER2/neu levels in a trastuzumab-based neoadjuvant setting in 16 patients. In this small group of patients, they could show that a decrease of serum HER2/neu levels was associated with response to therapy. In a study with 39 patients with neoadjuvant treatment, and 29 patients receiving a trastuzumab combination, Mazouni et al. [12] could only find a role of decreasing serum HER2/neu levels in prediction therapy response from weeks 3 to 6 after therapy initiation.
In our previous study, we investigated the incidence of elevated serum HER2/neu in primary breast cancer and metastatic breast cancer, and the correlation between status of tissue HER2/neu and serum HER2/neu [13]. Then we aimed at studying: first, the changes of serum HER2/neu before neoadjuvant chemotherapy and after neoadjuvant chemotherapy, second, the correlation between the change of serum HER2/neu and pathologic response, and third, the correlation between chemotherapy regimen and the change of serum HER2/neu.
We included 21 localized advanced breast cancer patients with informed consent of neoadjuvant chemotherapy who were treated at a single institute. Clinicopathologic features such as age, menopausal status, lymph node status (ycN, ypN), tumor status (ycT, ypT), estrogen receptor and progesterone receptor status, and HER2 (0, 1+, 2+ without gene amplification/2+ with gene amplification, 3+) were evaluated. We performed tissue immunohistochemical stain (IHC), and then HER2/neu 2+ expressed breast cancers examined by fluorescent in situ hybridization (FISH) analysis on tissue samples. We used the following neoadjuvant chemotherapies: 1) anthracycline-based regimen (A) (doxorubicin+cyclophosphamide) with 7 patients; 2) doxorubicin+cyclophosphamide→docetaxel with 2 patients; 3) docetaxel+doxorubicin with 8 patients, 4) a trastuzumab-based regimen (H) (trastuzumab+docetaxel) with 2 patients; and 5) trastuzumab+gemcitabine+paclitaxel with 3 patients. All serum samples were obtained twice: on the first day of chemotherapy and 3 weeks after the fourth neoadjuvant chemotherapy cycles were finished.
These patients cohort of the present study received one of the following neoadjuvant chemotherapies: doxorubicin (50 mg/m2, day 1) plus docetaxel (75 mg/m2, day 1) chemotherapy (AT) every 3 weeks for 6 cycles; doxorubicin (60 mg/m2, day 1) plus cyclophosphomide (600 mg/m2, day 1) chemotherapy (AC) every 3 weeks for 4 cycles; and doxorubicin (60 mg/m2, day 1) plus cyclophosphomide (600 mg/m2, day 1) chemotherapy (AC) every 3 weeks for 4 cycles, followed by docetaxel (100 mg/m2) every 3 weeks for 4 cycles. Trastuzumab (4 mg/kg, day 1) every week plus docetaxel (75 mg/m2) every 3 weeks for 4 cycles, and trastuzumab (4 mg/kg, day 1) every week plus paclitaxel (175 mg/m2) plus gemcitabine (1,250 mg/m2) every 3weeks for 4 cycles.
Clinical status was determined by available clinical data in the form of physical examination and imaging techniques (ultrasound, X-ray, computed tomography, magnetic resonance imaging) at the second and forth visit. All patients' responses to treatment were assessed according to the criteria of response evaluation criteria in solid tumor (RECIST) [14]. The clinical status in terms of whether the response was complete or partial and whether the disease was stable or progressive was determined. Complete or partial response was confirmed at a minimum of 4 weeks after at least 4 cycles (4 to 8 cycles) of chemotherapy were completed. And then, patients underwent surgical treatments.
Parraffin-embedded tissues was cut into 4-µm sections and mounted on glass slides. The sections were deparaffinized in xylene and rehydrated through alcohols to distilled water. Sections were incubated for 15 minutes in a 10-mmol/L citrate buffer, Ph 6.0, at 98℃. Polyclonal rabbit antihuman c-erbB-2 oncoprotein (Dako, Glostrup, Denmark) was applied at 1:100 dilutions. The slides were stained in an ES automatic stainer (Ventanna, Tucson, USA), according to the method recommended for the Dako HercepTest (Dako).
Parraffin-embeded tissue was cut into 4-µm sections, mounted onto positively charged slides, baked at 56℃ overnight, and deparaffinized. FISH analysis was performed using a Path HER-2 DNA Probe Kit (Vysis Inc., Downers Grove, USA) according to the manufacturer's recommendations. The tissues were briefly pretreated and were subjected to protease digestion. A hybridization solution containing directly labeled probes of Spectrum Green for chromosome 17centromere (CEP) and Spectrum Orange for HER2 gene locus were applied, and the denaturation and hybridization procedures were performed in a HYBrite system (Vysis). The slides were washed in a 2×SSC BUFFER (0.3 mol/L NaCl and 0.03 mol/L sodium citrate; Ph 7.2) at 72℃ for 2 minutes and counterstained with diaminopyrolylinodole. Two observers assessed each slide with a Nikon Eclipse E600 fluroscence microscope equipped with narrow band-pass filters. The observers scored at least 60 cells per specimen for HER2/neu (ERBB2) and CEP signals. We set a HER2:CEP ratio of >2.0 as evidence of HER2 (ERBB2) amplification.
Blood was drawn into serum separator tubes and was centrifuged at 2,000 g for 10 minutes at room temperature. Serum samples were stored at -70℃ until used. To measure the serum HER2 ECD concentration, we used a 2-site chemiluminescence sandwich immunoassay (ADVIA Centaur System; Bayer Diagnostics, Tarrytown, USA) with a detection limit of 0.5 µg/L; the interassay CV in our laboratory was 5.9%. Following the manufacturer's recommendation, we used 15 µg/L as the serum HER2 ECD cut-off to discriminate normal concentrations from those in patients with breast cancer. Serum HER2 ECD measurement was carried out in a blinded manner without knowledge about the results of IHC and FISH tests. When clinically indicated, we measured CA15-3 using a cut-off value of 30 units/mL. In the current study, we used the serum HER2/neu test with the Bayer ImmunoI® Automated system. In the report by Cook et al. [15], the cut-off value was 15 ng/mL, and the specificity for benign breast disease and other benign non-breast disease was 98.0% and 94.6%, respectively. But we considered the cut-off value as 10.2 ng/mL according to the Korean breast cancer patients [13].
We used the SPSS version 12.0 software (SPSS Inc., Chicago, USA) in statistical analysis. To examine the relationship between pathologic response and change of serum HER2/neu (postchemotherapy-prechemotherapy) or serum HER2/neu in prechemotherapy, we used the t-test and chi-square test to compare the clinicopathologic value and chemotherapy regimens with pathologic response. The Mann-Whitney non-parametric t-test was used to evaluate the significance of difference in serum HER2/neu concentrations between pathologic response separated by treatment groups. Sensitivities were estimated for a specificity of 95%, by ranking the predicted fit for each control subject and determining the cut-off values corresponding to these levels of specificity. A p-value of <0.05 was considered significant.
Response to neoadjuvant chemotherapy as defined by regression stable disease (SD), partial response (PR), complete response (CR), progressive disease (PD) was found in the following frequencies: SD and PD, each 0 (0%) of tumors; PR, 16 (77.3%); CR, 5 (22.7%) (Table 1). There was no statistically significant correlation between histolopathologic response and tissue HER2/neu status categories with score 0, 1+, and 2+, without gene amplification, and with scores 3+ and 2+, with gene amplification and estrogen and progesterone receptor status and ycT stage, ypT stage, ycN stage, and ypN stage (Table 2). No statistically significant correlation was found between prechemotherapy serum HER2/neu level and menopausal status (premenopausal, 15.5 ng/mL; postmenopausal, 14.4 ng/mL, p=0.91).
Mean of serum HER2/neu in prechemotherapy was 15.4 ng/mL (SD=9.0); that of postchemotherapy was 10.5 ng/mL (SD=2.0), and (p=0.04). In patients with complete pathologic response (pCR) of invasive tumor, the median prechemotherapy serum HER2/neu value was 23.7 ng/mL (±13.1); in patients with PR, it was 11.7 ng/mL (±2.2) (Figure 1). The median pretreatment serum HER2/neu level in patients with pCR was significantly higher than that in patients with partial response (p=0.01). The change of serum HER2/neu value was significantly higher than that in patients with CR (pCR, 13.2±14.1; PR, 2.06±1.3; p=0.02) (Table 2, Figure 2). According to the cut-off value (10.2 ng/mL), the change of prechemotherapy serum HER2/neu from the above cut-off value to below that was correlated with complete pathologic response (p=0.04).
Serum HER2/neu of the trastuzumab group showed a greater decreases than those of the anthracycline group (H=12.9± 14.5 ng/mL; A=2.2±1.2 ng/mL; p=0.02) during chemotherapy (Table 2). Figure 3 represents the receiver operating characteristics (ROC) curve for pathologic response as prechemotherapy serum HER2/neu concentration. The area under the curve is 0.827 (95% confidence interval [CI], 0.59-1.055; p=0.04). This value of the area under the curve indicates that the variable discriminates between those patients whose disease responded and those whose disease did not respond. Examination of the coordinates of the curve indicates that an optimal cut-off point of the value is 12.15 ng/mL. At this value, the sensitivity is 82.7%, and the specificity of 54.5%. Figure 4 represents the ROC curve for pathologic response as the change of serum HER2/neu concentration. The area under the curve is 0.782 (95% CI, 0.48-1.07; p=0.079).
Serum HER2/neu of the trastuzumab group decreased more than that of the anthracyline group (H=12.9±14.5 ng/mL; A=2.2±1.2 ng/mL; p=0.02). In the trastuzumab group, pCR was on the borderline, and significantly correlated with the change of serum HER2/neu (PR, 0.8±0.84 ng/mL vs. pCR, 21.1±13.2 ng/mL; p=0.08).
A growing number of investigators have begun to utilize the preoperative nature of neoadjuvant chemotherapy to conduct a treatment analysis of molecular markers that may predict response to therapy, and the emergence of new technologies such as transcriptional and proteomic profiling has greatly aided such investigations. Pallud et al. [16] found that with HER2/neu expression in paired serum and tissue samples, elevated serum HER2/neu concentration was related only to pT (p=0.0008), histological grade (p=0.0465); the presence of comedonecrosis (p=0.0123); or the comedo-type carcinoma (p=0.041), and that in these cases, the threshold in size and HER2/neu overexpression is necessary to observe elevated concentration of HER2/neu at diagnosis. Saghatchian et al. [17] reported that serum HER2/neu evaluation in early breast cancer correlated with the principal criteria of tumor aggressiveness, and thus that serum HER2/neu evaluation could be useful in identifying patients with a high risk of visceral metastases and contralateral breast tumors. Post-treatment serum HER2/neu was an independent prognostic factor enabling the identification of patients likely to benefit from aggressive adjuvant treatments.
In metastatic breast cancer patients, elevated serum HER2/neu levels are associated with decreased overall survival. With regard to progression-free survival, patients with high serum HER2/neu levels, in particular, seem to benefit from taxane containing chemotherapy. Colomer et al. [18] reported that in 58 patients with metastatic breast cancer treated with doxorubicin and paclitaxel, circulating HER2/neu ECD levels correlated inversely with radiologic response to therapy. In another study including 103 patients treated with epirubicin and cyclophosphamide or paclitaxel for metastatic breast cancer, no significant correlation between radiologic response to chemotherapy and serum HER2/neu levels was observed for the entire study population [19].
In this study, patients with metastatic breast cancer were selected for trastuzumab-based therapy if the HER2/neu protein was overexpressed (immunohistochemistry score 3+) in the primary tumor, or if FISH provided evidence of HER2/neu gene amplification. Although the HER2/neu ECD assay is approved by the Food and Drug Administration (FDA) for monitoring patients undergoing systemic therapy for metastatic breast cancer, the clinical utility of this marker in patients undergoing trastuzumab-based therapy is not well defined. In 2000, the American Society of Clinical Oncology Tumor Markers Expert Panel did not recommend the use of CEA, CA15-3, CA27-29 or HER2/neu ECD to monitor patients with metastatic breast cancer [20].
At the time of the report of the expert panel of the American Society of Clinical Oncology, there were no published data regarding circulating HER2/neu ECD and response to trastuzumab-based therapy. Since then, a few preliminary studies have found that serum HER2/neu followed the course of the disease while patients were receiving trastuzumab monoclonal antibody therapy. Kostler et al. [11] evaluated the clinical utility of serum HER2/neu in the early prediction of response to trastuzumab-based therapy in 55 patients with tissue HER2/neu-over-expressing metastatic breast cancer. The measurement of serum HER2/neu concentration is not exempted from the pathological determination of HER2/neu expression in tumors; some HER2/neu negative tumors are associated with high levels of serum HER2/neu; whereas, some HER2/neu positive tumors present low levels of serum HER2/neu. The concentration of serum HER2/neu is correlated to disease progression, since 10% of locoregional breast cancers and more than a third of metastatic breast cancers have high levels of serum HER2/neu. The elevation of serum HER2/neu levels generally occurs several months before the diagnosis of symptomatic metastasis.
The relevance of serum HER2/neu measurements during chemotherapy, hormone therapy, and trastuzumab treatment remains uncertain. In metastatic breast cancer, no clear relationship was found between baseline serum HER2/neu levels and tumor response to trastuzumab-based treatment in a recently published meta analysis, while other groups have suggested a role of serum HER2/neu determination such that no definitive conclusions can be drawn [21,22].
Similarly, it is not commonly agreed that high levels of serum HER2/neu before any treatment in the case of early breast cancer is an independent prognostic factor. There is limited information regarding the use of serum HER2/neu to predict benefit from trastuzumab treatment in primary breast cancers. None of the recent adjuvant trastuzumab trials have published an analysis of serum HER2/neu [23].
In neoadjuvant chemotherapy, previous studies have examined the value of biomarkers, such as carcinoembryogenic antigen, CA 15-3, MMP-2, MMP-9; tissue polypeptide antigen (TPA); tissue polpypetide-specific antigen (TPS); EGFR, and HER2/neu in predicting clinical or pathologic response to chemotherapy for breast cancer [24,25]. But the results of these investigations have been mixed and have not found the specific biomarker for each chemotherapy regimen.
We demonstrated a significant correlation of serum HER2/neu levels and histopathologic response of breast cancer to anthracycline-based and trastuzumab-based neoadjuvant chemotherapy. Schippinger et al. [26] demonstrated a statistically significant correlation of pretreatment serum HER2/neu and response to neoadjuvant anthracycline-based chemotherapy in breast cancer. ROC curve analysis revealed a serum HER2/neu value of more than 10.3 ng/mL to predict a pCR with 80% sensitivity and 69.4% specificity. Nolen et al. [27] demonstrated that a logistic regression analysis of the serum biomarker levels consisting of HER2/neu, EGFR, MIF, MMP-2, and CD 40 L that could distinguish responders from non-responders with 85% sensitivity and 69% specificity. In this study, a pretreatment serum HER2/neu value of more than 12.15 ng/mL predicted a pCR with 82.7% sensitivity and 54.5% specificity. It is noteworthy to mention that our present study identified a significant correlation between pretreatment serum HER2/neu or the change of serum HER2/neu and response to neoadjuvant chemotherapy, especially in the case of each chemotherapy regimens. This observation adds to several sparse and consistent reports in the literature [28].
Within the GepartQuattro trial, median prechemotherapy serum HER2/neu levels were significantly higher in patients with pCR than in patients without pCR, and median decrease of serum HER2/neu levels during therapy was also higher in patients with pCR [29].
In contrast to our findings, in the series reported by Mazouni et al. [12] in the neoadjuvant setting, mean serum HER2/neu baseline values were not different between the pCR group and the group with residual disease. Data from 63 studies were examined. Some studies showed significant association between raised concentration and poor prognosis; whereas, others did not. Examination of existing data showed that concentrations of serum HER2/neu ECD are not consistently related to patient outcomes; therefore, there is insufficient evidence to support the clinical use of serum HER2/neu ECD testing [30].
Although this study's population was too small to present the predictive ability of serum HER2/neu, the benefits of a serum biomarker, especially based chemotherapy regimen, are clearly illustrated, and further studies using larger clinical cohorts would be well warranted.
Figures and Tables
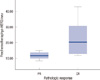 | Figure 1Correlation between the prechemotherapy serum HER2/neu concentration and pathologic response.
PR=partial response; CR=complete response.
|
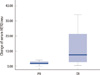 | Figure 2Correlation between the change of serum HER2/neu concentration before and after neoadjuvant chemotherapy and pathologic response.
PR=partial response; CR=complete response.
|
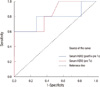 | Figure 3The receiver operating characteristics (ROC) curve analysis for prediction of pathologic response in 22 patients. Of these patients, 5 were treated with trastzumab in combination with taxane, and 17 with anthracycline. The curve shows pathologic response as a function of a prechemotherapy HER2/neu concentrations. The area under the curve is 0.829 (95% CI, 0.59-1.055). |
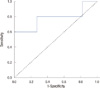 | Figure 4The receiver operating characteristics (ROC) curve analysis for prediction of pathologic response in 22 patients. Of these patients, 5 were treated with trastzumab in combination with taxane, and 17 patients with anthracycline. The curve shows pathologic response as a function of the change of serum HER2/neu concentrations before and after neoadjuvant chemotherapy. The area under the curve is 0.782 (95% CI, 0.48-1.07). |
Table 1
Patient's characteristics

ycT=prechemotherapy status clinical T stage; ycN=prechemotherapy status clinical N stage; ypT=postchemotheraphy status pathological T stage; ypN=postchemotheraphy status pathological N stage; ER=estrogen receptor; PR=progesterone receptor; CTx=chemotherapy; A=anthyracycline; C=cyclophosphamide; D=docetaxel; H=trastuzumab; P=paclitaxel; G=gemcitabine; N=negative; P=positive; CR=complete response; PR=partial response.
References
1. Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985. 230:1132–1139.

2. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987. 235:177–182.

3. Agrup M, Stål O, Olsen K, Wingren S. C-erbB-2 overexpression and survival in early onset breast cancer. Breast Cancer Res Treat. 2000. 63:23–29.

4. Zabrecky JR, Lam T, McKenzie SJ, Carney W. The extracellular domain of p185/neu is released from the surface of human breast carcinoma cells, SK-BR-3. J Biol Chem. 1991. 266:1716–1720.

5. Narita T, Funahashi H, Satoh Y, Takagi H. C-erbB-2 protein in the sera of breast cancer patients. Breast Cancer Res Treat. 1992. 24:97–102.

6. Molina R, Jo J, Filella X, Zanon G, Pahisa J, Muñoz M, et al. c-erbB-2 oncoprotein, CEA, and CA 15.3 in patients with breast cancer: prognostic value. Breast Cancer Res Treat. 1998. 51:109–119.

7. Fehm T, Maimonis P, Weitz S, Teramoto Y, Katalinic A, Jäger W. Influence of circulating c-erbB-2 serum protein on response to adjuvant chemotherapy in node-positive breast cancer patients. Breast Cancer Res Treat. 1997. 43:87–95.

8. Leitzel K, Teramoto Y, Konrad K, Chinchilli VM, Volas G, Grossberg H, et al. Elevated serum c-erbB-2 antigen levels and decreased response to hormone therapy of breast cancer. J Clin Oncol. 1995. 13:1129–1135.

9. Yamauchi H, O'Neill A, Gelman R, Carney W, Tenney DY, Hösch S, et al. Prediction of response to antiestrogen therapy in advanced breast cancer patients by pretreatment circulating levels of extracellular domain of the HER-2/c-neu protein. J Clin Oncol. 1997. 15:2518–2525.

10. Paik S, Bryant J, Park C, Fisher B, Tan-Chiu E, Hyams D, et al. erbB-2 and response to doxorubicin in patients with axillary lymph node-positive, hormone receptor-negative breast cancer. J Natl Cancer Inst. 1998. 90:1361–1370.

11. Kostler WJ, Schwab B, Singer CF, Neumann R, Rücklinger E, Brodowicz T, et al. Monitoring of serum Her-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancer. Clin Cancer Res. 2004. 10:1618–1624.

12. Mazouni C, Hall A, Broglio K, Fritsche H, Andre F, Esteva FJ, et al. Kinetics of serum HER-2/neu changes in patients with HER-2-positive primary breast cancer after initiation of primary chemotherapy. Cancer. 2007. 109:496–501.

13. Kong SY, Kang JH, Kwon Y, Kang HS, Chung KW, Kang SH, et al. Serum HER-2 concentration in patients with primary breast cancer. J Clin Pathol. 2006. 59:373–376.

14. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.
15. Cook GB, Neaman IE, Goldblatt JL, Cambetas DR, Hussain M, Lüftner D, et al. Clinical utility of serum HER-2/neu testing on the Bayer Immuno 1 automated system in breast cancer. Anticancer Res. 2001. 21(2B):1465–1470.
16. Pallud C, Guinebretiere JM, Guepratte S, Hacene K, Neumann R, Carney W, et al. Tissue expression and serum levels of the oncoprotein HER-2/neu in 157 primary breast tumours. Anticancer Res. 2005. 25(2B):1433–1440.
17. Saghatchian M, Guepratte S, Hacene K, Neumann R, Floiras JL, Pichon MF. Serum HER-2 extracellular domain: relationship with clinicobiological presentation and prognostic value before and after primary treatment in 701 breast cancer patients. Int J Biol Markers. 2004. 19:14–22.

18. Colomer R, Montero S, Lluch A, Ojeda B, Barnadas A, Casado A, et al. Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin Cancer Res. 2000. 6:2356–2362.
19. Müller V, Witzel I, Lück HJ, Köhler G, von Minckwitz G, Möbus V, et al. Prognostic and predictive impact of the HER-2/ neu extracellular domain (ECD) in the serum of patients treated with chemotherapy for metastatic breast cancer. Breast Cancer Res Treat. 2004. 86:9–18.

20. Bast RC Jr, Ravdin P, Hayes DF, Bates S, Fritsche H Jr, Jessup JM, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001. 19:1865–1878.

21. Lennon S, Barton C, Banken L, Gianni L, Marty M, Baselga J, et al. Utility of serum HER2 extracellular domain assessment in clinical decision making: pooled analysis of four trials of trastuzumab in metastatic breast cancer. J Clin Oncol. 2009. 27:1685–1693.

22. Ali SM, Leitzel K, Lipton A, Carney WP, Kostler WJ. Value of serum human epidermal growth factor receptor 2 (HER2)/neu testing for early prediction of response to HER2/neu-directed therapies is still an open one and deserves further study in large prospective trials. J Clin Oncol. 2009. 27:e273.
23. Leary AF, Hanna WM, van de Vijver MJ, Penault-Llorca F, Rüschoff J, Osamura RY, et al. Value and limitations of measuring HER-2 extracellular domain in the serum of breast cancer patients. J Clin Oncol. 2009. 27:1694–1705.

24. Coskun U, Yamac D, Gulbahar O, Sancak B, Karaman N, Ozkan S. Locally advanced breast carcinoma treated with neoadjuvant chemotherapy: are the changes in serum levels of YKL-40, MMP-2 and MMP-9 correlated with tumor response? Neoplasma. 2007. 54:348–352.
25. Martínez-Trufero J, de Lobera AR, Lao J, Puértolas T, Artal-Cortés A, Zorrilla M, et al. Serum markers and prognosis in locally advanced breast cancer. Tumori. 2005. 91:522–530.

26. Schippinger W, Dandachi N, Regitnig P, Hofmann G, Balic M, Neumann R, et al. The predictive value of EGFR and HER-2/neu in tumor tissue and serum for response to anthracycline-based neoadjuvant chemotherapy of breast cancer. Am J Clin Pathol. 2007. 128:630–637.

27. Nolen BM, Marks JR, Ta'san S, Rand A, Luong TM, Wang Y, et al. Serum biomarker profiles and response to neoadjuvant chemotherapy for locally advanced breast cancer. Breast Cancer Res. 2008. 10:R45.

28. Tiezzi DG, Andrade JM, Ribeiro-Silva A, Zola FE, Marana HR, Tiezzi MG. HER-2, p53, p21 and hormonal receptors proteins expression as predictive factors of response and prognosis in locally advanced breast cancer treated with neoadjuvant docetaxel plus epirubicin combination. BMC Cancer. 2007. 7:36.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download