Abstract
Purpose
Estrogen, through its binding to nuclear estrogen receptor (ER), has been implicated in the development of human breast cancer. The presence or absence of ER in breast lesions has been used to classify breast cancer into ER+ or ER- type. Maspin, an anti-breast cancer protein produced in normal mammary cells, has also been reported to control the condition. Studies have been conducted to determine the role of ER+ and ER- status in neutrophils in the synthesis of maspin in human breast cancer.
Methods
Maspin presence was determined by enzyme linked immunosorbent assay, while nitric oxide (NO) level was determined using the methemoglobin method.
Results
Scatchard plots of the equilibrium binding of estrogen demonstrated the presence of 4.18×107 receptors per normal neutrophil and 2.46×107 receptors per ER+ neutrophil with a similar dissociation constant (0.926 nM). The ER- type showed nonspecific estrogen binding only. At 0.6 nM estrogen, NO synthesis was maximally increased to 1.829 and 0.887 µM NO/109 cells at 4 hours in normal and ER+ neutrophils respectively, with synthesis of 2.383 and 1.422 nM maspin in normal and ER+ neutrophils respectively. Estrogen failed to produce these effects in ER- neutrophils.
Conclusion
ER status in neutrophils determined maspin synthesis in breast cancer through the stimulation of NO synthesis. Neutrophils with ER- status which do not produce any maspin when treated with estrogen, might imply a worse prognostic outcome in ER- breast cancer due to the lack of anti-breast cancer protein synthesis.
The most frequently encountered malignancy of breast cancer tissue in women represents a classical model of estrogen dependent condition [1]. However, the effects of estrogen, like the other steroid hormones, are reported to be mediated due to their ability to cross the lipid bilayer membrane of cells and to bind the nuclear receptors [2], which in turn are reported to interact with a specific DNA sequence known as hormone responsive elements (HRE). The binding of the receptors to the HRE is reported to lead to the expression of the hormonal effects [2]. On the basis of presence of the nuclear estrogen receptors (ER), patients with breast cancer are classified into two groups: ER+ and ER-.
As in the case of estrogen, which is known to play a significant role in the development of human breast cancer, an anti-breast cancer protein called maspin (mammary serine proteinase inhibitor, 42 kDa) which is abundantly expressed in the normal mammary epithelial cells is reported toinhibit malignant breast cell invasion, promote apoptosis, and inhibit angiogenesis, and metastasis [3-5]. Studies have been conducted to determine the correlation between ER function and maspin synthesis if any, in the systemic development of breast cancer in female subjects, but the answer remains obscure.
We report herein the impairment of ER function in the synthesis of maspin through the impairment of estrogen-induced stimulation of nitric oxide (NO) synthesis in circulating neutrophils in the presence of human breast cancer in which the ER status of the malignant lesion was identical to that present in the patients' neutrophils.
The protocol used in the study was approved by the Internal Review Board (IRB) of Sinha Institute of Medical Science and Technology. All of the patients with breast cancer and the age-matched normal female volunteers provided informed consent. Appropriate permission was also obtained from the IRB (approval no. 1/8/R/Br) for the use of rabbits in the studies.
Recombinant human maspin (rh maspin) was a kind gift of Dr. Sally Twining (Department of Biochemistry, Medical College of Wisconsin, Milwaukee, USA). The enzyme linked immunosorbent assay (ELISA) maxisorp plates were obtained from NUNC (Roskilde, Denmark). The estrogen and all other chemicals used were from Sigma Chemical Co. (St. Louis, USA). ERα and ERβ antibodies were obtained from Thermo Fisher Scientific (Rochester, USA).
Estrogen solution was prepared by dissolving the compound in 0.9% NaCl, and the pH was then adjusted to 7.4. The steroid hormone solution was used immediately, after preparation and discarded after use.
Only female breast cancer patients or normal age-matched female volunteers 35 to 65 years of age (mean, 45 years; n=50), participated in the study. The occurrence of breast cancer was diagnosed by mammography or biopsy. None of the patients had received any therapy including radiation, surgery, or chemotherapy but were waiting for surgical intervention. These patients at presentation were staged as follows: Fifteen patients were staged at IIA (T1N1 M0), 20 patients were staged at IIB (T2N1 M0), 10 patients were staged at IIIA (T3N2 M0), and five patients were staged at IIIB (T4N0 M0). All subjects were M0. None of the subjects had a history of diabetes mellitus, systemic hypertension, severe infections, or life threatening cardiovascular or cerebrovascular conditions.
An equal number of age-matched normal female volunteers in similar phases of their menstrual cycles as those of the selected breast cancer subjects were asked to participate in the study. These volunteers had never taken any contraceptives. All volunteers were asked to stop taking any medication including aspirin for at least 2 weeks before participating in the study.
Blood samples (20-25 mL) were collected by venipuncture using siliconized 19-gauge needles in plastic vials, and anticoagulated by gentle mixing of 1 volume of 0.13 M sodium citrate with nine volumes of blood [6].
Polyclonal antibodies against rh maspin were raised by repeated immunization of White New Zealand rabbits as described previously [7].
Neutrophils were isolated from the citrated blood samples as described previously [9]. The cell counts were determined using optical microscopy. The isolated neutrophils suspended in Hank's balanced salt solution (HBSS), pH 7.4, (6×109 cells/L) were incubated with different concentrations of estrogen as indicated for 4 hours at 37℃ under sterile conditions, and when needed, the nucleic acids were isolated from these incubated samples for in vitro translation of maspin as described below.
Nucleic acids containing maspin mRNAs were isolated using the TRIzol method in neutrophils isolated from the blood samples [10]. The nucleic acid preparation was incubated with ribosomal preparation, a mixture of all amino acids (0.1 µmol each/mL) and 2 mM adenosine triphosphate as described previously [11]. After 6 hours of incubation under sterile conditions, the reaction mixture at 0℃ was centrifuged at 10,000×g for 10 minutes. The supernatant was used for the determination of maspin by ELISA as described below.
Isolated neutrophils were placed on glass slides, and frozen using cold liquid nitrogen vapor, and broken by the sliding of another glass slide over them to expose the nuclear receptors in the cells to the added fluorescent antibody. ER statuses were determined by immunohistochemical techniques using fluorescence tagged antibodies that recognized both α and β estrogen receptors [13]. The cells were then immediately observed and photographed under fluorescence microscopy.
In preliminary experiments to determine the optimal time for estrogen binding, the normal neutrophil suspensions (6×109 cells/L) were incubated with 0.1 to 1.0 nM estrogen for different time periods at 37℃. The amounts of estrogen that bound to the neutrophils were determined after unbound hormone was separated from the bound hormone in the incubation mixture using ELISA as described below in the Scatchard plot analysis of estrogen binding.
The neutrophil suspensions were prepared from the blood samples from normal or from the subjects with breast cancer and suspended (6×109 cells/L) in HBSS buffer, pH 7.4, with different amounts of pure estrogen and incubated for different periods of time at 37℃. After incubation, the neutrophils with the bound estrogen were separated from the unbound hormone by filtration over a glass microfiber filter (GF/C; Sigma Chemical Co., St. Louis, USA) using a Millipore filter as described previously [14]. After filtration, the neutrophils were washed twice with equal volumes of HBSS buffer. The GF/C filter that retained the neutrophils with the bound hormone was subsequently air dried and estrogen was eluted from the filter by trituration with 1 mL of a CHCl3 CH3OH (1:1) mixture. After centrifugation at 0℃ and 5,000×g, portions of the supernatant were air dried. The air-dried sample was redissolved in 0.9% NaCl and its estrogen concentration was determined using ELISA. The results obtained were further verified using 1.0 µci (4-14C) estradiol (Tjaden Biosciences, Burlington, USA) to the incubation mixture. The bound estrogen was separated from the unbound ligand as described above and the radioactivity was measured to determine the binding using a scintillation counter as described previously [14].
Specific estrogen binding was determined by the addition of 10 mM unlabeled estrogen to the radio labeled estradiol as described above after subtracting the nonspecific binding from the total binding. The dissociation constant (Kd) and the receptor numbers (n) from the Scatchard plots [15] were determined by computer analysis.
In some study phases, the normal neutrophils suspension was incubated separately with ERα antibody or ERβ antibody or both in HBSS buffer as described above for 1 hour at 37℃ and the binding of estrogen was subsequently determined.
The rate of binding of (4-14C) estradiol to the normal neutrophils was determined after 1 hour of incubation (i.e., at the steady state of the binding). The bound 14C estradiol to the neutrophils was separated from the unbound compound via GF/C membrane filtration as described above. The radioactivity of the bound estrogen was then determined as described under Scatchard plots.
The obtained results are presented as mean±SD, while the significance of the results was determined using Student's t-test. Values of p<0.005 were considered significant. Where appropriate, the coefficient of correlation (r) of the results was also determined using the, Pearson test. GraphPad Prism software (GraphPad Software, San Diego, USA) was used for the statistical analyses.
Immunohistochemical studies of the ER+ status in breast tissue and in the normal peripheral neutrophils demonstrated the presence of both ERα and ERβ subtypes. In contrast, ER- neutrophils from patients with breast cancer lacked the ERα and ERβ subtypes, suggesting similar pathophysiological defects in the synthesis of ER (α and ER β) proteins in both malignant breast tissues and peripheral neutrophils that were shown by histopathology to be identical to the malignant breast lesion subtype of the victim (Figure 1).
Incubation of normal, ER+, and ER- neutrophils with 0.6 nM estrogen (the optimal estrogen concentration for maspin synthesis as determined in earlier experiments) produced saturable and specific binding profiles of the hormone to the neutrophils after 4 hours incubation at 37℃ at equilibrium (Figure 2). The amounts of estrogen bound to the ER+ neutrophils were, however, found to be markedly lower than those in the normal cells. In contrast, similar treatment of ER- neutrophils with estrogen showed little or no specific hormone binding, a finding that was similar to the nonspecific binding of the hormone to normal neutrophils (Figure 2).
A Scatchard plot of the equilibrium binding of estrogen to normal neutrophils produced a typical linear profile representing a homogeneous ER population within the neutrophils (Figure 3). Analysis of the equilibrium binding characteristics of estrogen demonstrated that the ER number in normal neutrophils was 4.18±1.02×107 sites/cell (Kd)=of 0.926 nM. A Scatchard plot of the equilibrium binding of estrogen to ER+ neutrophils also demonstrated a linear profile (Figure 3) similar to the normal neutrophil result. However, analysis of the hormone binding characteristics to ER+ neutrophils in the Scatchard plot showed that the ER count was nearly half (2.46±0.025×107 estrogen binding sites/cell) of that in normal neutrophils described above (p<0.001, n=5). However, the dissociation constants were identical between ER+ and normal neutrophils (Kd=0.926 nM). Since ER- neutrophils did not demonstrate estrogen binding, it was not possible to determine the ER number or the dissociation constant under the experimental conditions described in the Methods section.
Incubation of the normal neutrophils suspension in HBSS buffer, pH 7.4, for 4 hours with different amounts of estrogen as indicated at 37℃ resulted in NO synthesis in the incubation mixture (Figure 4). Both normal and ER+ neutrophils from the patients with breast cancer synthesized the most detectable NO at 0.6 nM estrogen. However, similar treatment of ER- neutrophils with different amounts of estrogen failed to produce any detectable amounts of NO in the reaction mixture under otherwise identical conditions.
We also found that normal neutrophils, which contained higher numbers of estrogen binding sites (4.18±1.02×107 sites/cell) than did ER+ neutrophils (2.46±0.025×107 sites/cell), synthesized higher quantities of NO (1.829±0.072 µM) than those synthesized by ER+ neutrophils (0.887±0.003 µM) when treated with 0.6 nM estrogen (p<0.001, n=15) (Figure 4).
These results indicated that the occurrence of estrogen-induced NO synthesis at the optimal concentration of the (i.e., 0.6 nM) was related to the number of ER both in normal and ER+ neutrophils, which had identical hormone affinities (Kd=0.926 nM) in both instances. In contrast, ER- neutrophils, which showed no estrogen binding, did not produce any NO upon estrogen treatment (Figure 4).
The estrogen binding rate to normal neutrophils remained steady for 1 hour at 37℃ (19±0.12 nM/109 cells) but decreased to 17±0.11 nM/109 cells (11% inhibition) and 16±0.11 nM/109 cells (16% inhibition) upon incubation with ERβ and ERα antibody, respectively, as described in the Methods section. In contrast, when the normal neutrophils were incubated with both ERα and ERβ antibodies, estrogen binding decreased markedly (75%) to 0.5±0.11 nM/109 cells under otherwise identical conditions (Table 1).
Since increased cellular concentrations of NO have been reported to result in increased maspin synthesis in neutrophils [6], experiments were conducted to determine the effect of estrogen-induced increase of NO in neutrophils in the synthesis of maspin in normal, ER+, and ER- neutrophils (Figure 5). We found that normal neutrophils in which NO synthesis was maximally stimulated at 0.6 nM also synthesized maximal levels of maspin (2.383±0.014 nM). In contrast, in ER+ neutrophils, in which the estrogen-induced synthesis of NO was nearly half, of that in normal cells, the amount of maspin synthesis was reduced to 1.422±0.029 nM (p<0.005) (Figures 4 and 5).
In contrast to normal and ER+ neutrophils, treatment of ER- neutrophils with similar amounts of estrogen failed to produce NO, or maspin (Figure 5). The addition of L-NG Nitroarginine Methyl Ester (NAME), an NO synthase inhibitor [16], to normal or ER+ neutrophils resulted in complete inhibition of the estrogen-induced synthesis of both NO and maspin.
Furthermore, treatment of normal and ER+ neutrophils with 5 µM NO (final) solution in 0.9% NaCl, instead of estrogen, produced 2.90±0.17 nM and 1.95±0.13 nM maspin, respectively. Interestingly, although the treatment of ER- neutrophils with estrogen failed to produce any NO or maspin, the addition of NO solution to the ER- cells resulted in the synthesis of 0.87±0.005 nM maspin even in the absence of added estrogen to the incubation mixture (p<0.005). The addition of NAME to the reaction mixture containing NO for the synthesis of maspin had no effect on the synthesis of the anti-breast cancer protein. These results indicated that although NO was capable of inducing maspin synthesis in ER- neutrophils, the addition of NAME, an inhibitor of nitric oxide synthase, had no effect on NO or the synthesis of maspin in the presence of added NO.
In other words, NAME had no effect on maspin synthesis induced by added NO to the reaction mixture containing neutrophils (Figure 6).
The coefficient of correlation "r" between the estrogen-induced NO synthesis and maspin production was +0.98 and +0.90 in normal and ER+ neutrophils, respectively, indicating that NO and maspin synthesis in these cells were highly and positively correlated.
The results of the current study demonstrated that ER status of the neutrophils in the peripheral blood of the subjects with breast cancer, as determined by immunohistochemical cytology, was identical to those of the patients' breast lesions of the victims (Figure 1).The ER activity was also demonstrated to be impaired in that steroid induced maspin synthesis due to impaired NO production occurred in both ER+ and ER- human breast cancer neutrophils compared to normal neutrophils (Figures 4 and 5).
The defective hormone receptor synthesis in cells other than in breast cancer cells themselves is not, unique, in fact, impaired insulin receptor activity has also been reported to occur both in breast cancer cells and in erythrocytes in the circulation in this condition [6]. It should be mentioned here that insulin, like estrogen, was also a potent inducer of maspin synthesis through NO production [6].
Furthermore, these results indicated that the estrogen-induced synthesis of maspin in neutrophils either from normal female volunteers or from patients with ER+ breast cancer was an NO dependent post ER interaction phenomenon. Although the treatment of both normal or ER+ neutrophils with estrogen resulted in the stimulation of maspin synthesis through NO production, similar treatment of ER- neutrophils with the hormone failed to stimulate either NO or maspin synthesis under identical conditions.
It is generally believed that steroid hormones including estrogen, which are lipid soluble, can freely pass through cell membrane bilayers of the cells [17] and are and subsequently bound to the nuclear receptors in the cytosol that mediate the expression of specific genes in the cells [6]. However, if estrogen were able to freely diffuse through the neutrophil membrane due to its lipid solubility of the steroid alone, then the synthesis of either NO or maspin might occur equally in ER+ and ER- neutrophils as determined by the effect of estrogen in the intact neutrophil suspensions (Figures 4 and 5). However, this was not the case. In contrast, our results suggested that the transport of estrogen into the neutrophils was an ER dependent physiological event. In other words, the ERs played an essential role in the transport of the steroid hormone into the cell through the lipid membrane bilayers and the transportation process of estrogen was not necessarily due only to the lipid soluble nature of the steroid hormone. This inference was made due to immunohistological studies that indicated the presence of both ERα and ERβ in the normal and ER+ neutrophils and the absence of both receptor subtypes in ER- neutrophils (Figure 1). These results suggested that both ERα and ERβ could be involved in the transport of estrogen into neutrophils.
The above conclusion was also supported by the fact that the incubation of normal neutrophils with ERα, ERβ, or both antibodies inhibited estrogen binding. We found that although incubation of the normal neutrophils suspension with ERα antibody only blocked estrogen binding by 15%, incubation of these cells with ERβ antibody blocked the hormone binding by 11%. In contrast, incubation of the normal neutrophils with both ERα and ERβ together blocked the binding by 75%.
Although the above results suggested that both ERα and ERβ could play an essential role in the binding/transportation of estrogen in neutrophils, the role of ERα and ERβ in estrogen-induced maspin synthesis via NO production could be of lesser importance, at least in the case of estrogen-induced synthesis of maspin in neutrophils (Figure 5). It was found that while the treatment of normal neutrophils with 25 nM progesterone instead of 0.6 nM estrogen increased the NO synthesis from 0.544 to 1.317 µM with simultaneous increase of maspin from 1.36 to 2.33 nM maspin/109 cells, similar treatment of ER+ neutrophils with 25 nM progesterone increased the NO synthesis from 0.32 to 0.73 µM and increased maspin synthesis from 0.41 to 1.14 nM/109 cells (data not shown). It could, however, be argued that progesterone at higher concentrations was simply mimicking estrogen in vitro. The physiological level of progesterone is also known to be 80 fold higher than that of estrogen in women in the mid cycle of their menstruation periods.
Although the effects of progesterone and estrogen could represent similar effects of two different hormones in the synthesis of maspin via NO production, these results nevertheless might also suggest favorably comparable effects of these two steroid hormones in the control of the development of human breast cancer through NO induced maspin production in the system instead of antagonizing their effects in the control of the condition as has been reported previously [18].
It could be argued that the lack of maspin synthesis in ER- neutrophils was not due to the lack of estrogen receptors in the cells (Figures 4 and 5) but rather was due to mutation of the gene involved in the synthesis of the anti breast cancer protein in these neutrophils obtained from patients with breast cancer. However, the direct addition of NO solution to the ER- neutrophils that did synthesize maspin due to the presence of NO suggested that the estrogen-induced impairment of maspin synthesis in the ER- neutrophils might not be directly related to the mutation of the maspin gene because NO could not stimulate maspin synthesis in ER- neutrophils.
On the other hand, these results indicated that as a result of estrogen binding to its nuclear receptors, the synthesis of NO synthase occurred due to the nuclear receptor interaction with the HRE in the DNA of the neutrophils. NO formed due to the catalytic activity of the NO synthase produced by the neutrophils and, subsequently stimulated maspin synthesis. The ability of the formed NO to synthesize maspin in neutrophils has been reported before [6].
The inhibition of estrogen-induced NO synthase by NAME inhibited maspin synthesis. On the other hand, treatment of the neutrophils with NO solution resulted in maspin synthesis, of even in the presence of NAME or in the absence of estrogen. These results suggested that in the estrogen-induced maspin production due to the activation of ER that stimulated NO synthesis, the NO was acting like a "messenger" for maspin synthesis in neutrophils.
It has been reported previously that the prognostic outcome of breast cancer with ER- lesions was worse than that with ER+ lesions [19,20].
Our results, as presented above, suggested that estrogen-induced maspin synthesis was severely impaired in ER- neutrophils compared to that in ER+ neutrophils, as such, impaired the estrogen-induced maspin synthesis in patients with ER- breast cancer might result in worse prognostic outcome when the lesions are ER- status than when the lesions are ER+ status.
Figures and Tables
 | Figure 1The immunohistochemistry of estrogen receptors (ER) in neutrophils from normal volunteers and patients with breast cancer patients: normal (A), ER+ neutrophils (B), and ER- neutrophils (C). The Figure presented is representative of six or more experiments using neutrophils from six different subjects from each group. The immunohistochemistry of the ER was determined as described in the Methods section. The cells are observed under 45× objective. |
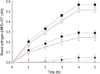 | Figure 2Equilibrium binding of estrogen to normal, estrogen receptor+ (ER+), and ER- neutrophils. Neutrophil suspensions prepared from normal, ER+, and ER- neutrophils were prepared from patients with breast cancer that were treated with 0.6 nM estrogen for different periods of time for equilibrium binding as indicated. The nonspecific binding was determined by adding excess amounts of estrogen (10 mM) to binding mixture containing 1.0 µCi of 14C estradiol as described in the Methods section. The levels estrogen bound to the neutrophils were determined by enzyme linked immunosorbent assay as described in the Methods section. Specific binding was calculated by subtracting the nonspecific binding from the total binding. The solid squares (■) and hollow squares (□) represent total binding and specific binding in normal neutrophils, respectively. The solid circles (●) and hollow circles (◯) indicate total and specific binding in ER+ neutrophils, respectively. The solid triangles (▲) and hollow triangles (△) represent the total and specific binding in neutrophils isolated from ER- subjects, respectively. The solid lines (  ) represent specific binding, while the dotted lines ( ) represent specific binding, while the dotted lines ( ) represent total binding of estrogen to the neutrophils. The results are the mean±SD of five different experiments each in triplicate using blood of five different patients with ER+ or ER- breast cancer or from normal volunteers. ) represent total binding of estrogen to the neutrophils. The results are the mean±SD of five different experiments each in triplicate using blood of five different patients with ER+ or ER- breast cancer or from normal volunteers. |
 | Figure 3Scatchard plots of the binding of estrogen to neutrophils of normal volunteers, and or patients with estrogen receptor+ (ER+) breast cancer. The neutrophils preparations were suspended in Hank's balanced salt solution (pH 7.4) containing different concentrations of estrogen for 4 hours as indicated for equilibrium binding. The total binding for each point was calculated from the total amount of the ligand present in the reaction mixture. The Scatchard plots shown here are representative of three experiments conducted for each group (n=5). The solid squares (■) represent a Scatchard plot of neutrophils from normal volunteers, while the solid circles (●) indicate a Scatchard plot using neutrophils from patients with ER+ breast cancer. |
 | Figure 4Effect of different amounts of estrogen on the synthesis of nitric oxide (NO) in normal, estrogen receptor (ER)+, or ER- neutrophils. The neutrophil suspensions were prepared from the blood of patients with ER+ or ER- breast cancer and from age-matched normal female volunteers as described in the Methods section (n=15, in each group). The neutrophils preparations (6×109 cells/L) were suspended in Hank's balanced salt solution (pH 7.4) and treated with different concentrations of estrogen (nM) as indicated. In a parallel set of experiments, neutrophil suspensions from age-matched normal female volunteers and from patients with ER+ or ER- breast cancer were incubated with NAME along with different amounts of estrogen. After incubation for 4 hours at 37℃ under sterile conditions, NO synthesis in the reaction mixture was determined using a methemoglobin assay. Solid circles (●) represent NO synthesis in normal neutrophils while, solid squares (■) indicate NO synthesis in ER+ breast cancer neutrophils, the solid triangles (▲) represent NO synthesis in ER- breast cancer neutrophils, and the hollow circles (◯) indicate NO synthesis in presence of NAME. Results are mean±SD of five different experiments in triplicate using blood of 15 different patients with breast cancer in each group and 15 normal female volunteers. NAME=L-NG Nitroarginine Methyl Ester. |
 | Figure 5Eeffect of different amounts of estrogen on maspin synthesis in normal, estrogen receptor (ER)+, and ER- neutrophils. Neutrophil suspensions were prepared from the blood of patients with ER+ or ERbreast cancer receptor status and from age-matched normal female volunteers as described in the Methods section (n=15, in each group). The neutrophil preparations (6×109 cells/L) were suspended in Hank's balanced salt solution (pH 7.4) and treated with different concentrations of estrogen (nM) as indicated. In a parallel set of experiments, neutrophil suspensions from age-matched normal female volunteers and from patients with ER+ or ER- breast cancer were incubated with NAME in the presence of varying amounts of estrogen. After incubation for 4 hours at 37℃, in vitro translation of maspin mRNAs was conducted and maspin synthesis in the reaction mixture was determined by enzyme linked immunosorbent assay as described in the Methods section. The solid circles (●) represent maspin synthesis in normal neutrophils, while the solid squares (■) indicate maspin synthesis in ER+ breast cancer neutrophils. The solid triangles (▲) represent maspin synthesis in ER- breast cancer neutrophils, while the hollow circles (◯) indicate maspin synthesis in the presence of NAME. Results are mean±SD of five different experiments in triplicate using blood of 15 patients with breast cancer and 15 normal female volunteers.
NAME=L-NG Nitroarginine Methyl Ester.
|
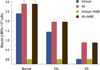 | Figure 6Effect of estrogen, nitric oxide (NO), and NAME on maspin synthesis in normal, estrogen receptor (ER)+, and ER- neutrophils. The neutrophil suspensions were prepared from the blood of patients with ER+ or ER- breast cancer and of age-matched normal female volunteers as described in the Methods section. The neutrophil preparations (6×109 cells/L) were suspended in Hank's balanced salt solution (pH 7.4) and treated with estrogen (0.6 nM) and, NO (5 µM). In a separate experiment, a neutrophil suspension was incubated with NAME (10 mM) and with either NO or estrogen. Results are mean±SD of five different experiments in triplicate using blood of 15 patients with breast cancer and 15 normal female volunteers.
NAME=L-NG Nitroarginine Methyl Ester.
|
Table 1
Effect of incubation of normal neutrophils with estrogen receptor α (ERα) or ERβ antibody on estrogen binding to the cells

Neutrophils were isolated from normal blood samples and suspended in Hank's balanced salt solution as described in the Methods section. The neutrophil suspensions were incubated separately with ERα, ERβ, or both antibodies for 1 hour at 37℃ to attain the steady state binding as indicated. Binding of the steroid hormone to the antibodies was subsequently determined. The results shown are the mean±SD of five different experiments each in triplicate using blood from five normal female volunteers.
ACKNOWLEDGEMENTS
This study was financially supported by donations from the families of breast cancer patients and by private donors.
References
1. Schneider HP, Jackisch C. Potential benefits of estrogens and progestogens on breast cancer. Int J Fertil Womens Med. 1998. 43:278–285.
3. Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994. 263:526–529.

4. Hojo T, Akiyama Y, Nagasaki K, Maruyama K, Kikuchi K, Ikeda T, et al. Association of maspin expression with the malignancy grade and tumor vascularization in breast cancer tissues. Cancer Lett. 2001. 171:103–110.

5. Liu T, Pemberton PA, Robertson AD. Three-state unfolding and self-association of maspin, a tumor-suppressing serpin. J Biol Chem. 1999. 274:29628–29632.

6. Girish GV, Bhattacharya G, Sinha AK. The role of insulin dependent NO synthesis in the impaired production of maspin in human breast cancer. J Cancer Res Clin Oncol. 2006. 132:389–398.

7. Tlaskalová-Hogenová H, Stépánková R. Development of antibody formation in germ-free and conventionally reared rabbits: the role of intestinal lymphoid tissue in antibody formation to E. coli antigens. Folia Biol (Praha). 1980. 26:81–93.
8. Cox RD, Frank CW. Determination of nitrate and nitrite in blood and urine by chemiluminescence. J Anal Toxicol. 1982. 6:148–152.

9. Klock JC, Bainton DF. Degranulation and abnormal bactericidal function of granulocytes procured by reversible adhesion to nylon wool. Blood. 1976. 48:149–161.

10. Cook L, Ross AM, Knight GB, Agnello V. Use of whole blood specimens for routine clinical quantitation of hepatitis C virus RNA does not increase assay sensitivity. J Clin Microbiol. 2000. 38:4326–4331.

11. Zimmerman R, Paluch U, Sprinzl M, Neupert W. Cell-free synthesis of the mitochondrial ADP/ATP carrier protein of Neurospora crassa. Eur J Biochem. 1979. 99:247–252.

12. Engvall E, Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972. 109:129–135.
13. Motomura K, Ishitobi M, Komoike Y, Koyama H, Nagase H, Inaji H, et al. Expression of estrogen receptor beta and phosphorylation of estrogen receptor alpha serine 167 correlate with progression-free survival in patients with metastatic breast cancer treated with aromatase inhibitors. Oncology. 2010. 79:55–61.

14. Kahn NN, Sinha AK. Stimulation of prostaglandin E1 binding to human blood platelet membrane by insulin and the activation of adenylate cyclase. J Biol Chem. 1990. 265:4976–4981.

15. Scatchard G. The attractions of proteins for small molecules and ions. Ann N Y Acad Sci. 1949. 51:660–672.

16. Sakuma I, Stuehr DJ, Gross SS, Nathan C, Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988. 85:8664–8667.

17. Whiting KP, Restall CJ, Brain PF. Steroid hormone-induced effects on membrane fluidity and their potential roles in non-genomic mechanisms. Life Sci. 2000. 67:743–757.

18. Mauvais-Jarvis P, Kuttenn F, Gompel A. Antiestrogen action of progesterone in breast tissue. Breast Cancer Res Treat. 1986. 8:179–188.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download