Abstract
We report a rare case of intraductal lipid-rich carcinoma of the breast with a component of glycogen-rich carcinoma. An impalpable tumor that was revealed by mammography and magnetic resonance imaging was excised. Histologic examination showed vacuolated neoplastic cells in the mammary ducts, and electron microscopy confirmed lipid droplets in the cytoplasm. The coexistence of glycogen-rich carcinoma was shown. Lipid-rich carcinoma that is coexistent with glycogen-rich carcinoma is rare, and most lipid-rich carcinomas are invasive. Intraductal lipid-rich carcinoma is difficult to detect without echography or mammography.
Normal epithelial cells of the mammary gland are able to synthesize lipids, carbohydrates, and proteins. Breast cancer is histologically classified into subtypes according to the substances that the carcinoma cells contain-such as lipid-rich carcinoma and glycogen-rich carcinoma, while the types of carcinoma are mainly ductal carcinoma and lobular carcinoma. Aboumrad et al. [1] reported the first case of mammary carcinoma that contained lipids in the cytoplasm, which was diagnosed as lipid-secreting carcinoma. Lipid-rich carcinoma is a rare mammary cancer, and glycogen-rich carcinoma is also relatively rare. To the best of our knowledge, only 1 other case of invasive mammary carcinoma with composite histologic findings of these 2 carcinomas has been described [2]. We encountered a case of intraductal carcinoma with the coexistence of these 2 rare subtypes of mammary carcinoma, and performed a histological and ultrastructural examination of the lesion.
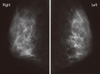
A Japanese woman in her fifth decade visited our hospital in order to be examined and medically treated for multiple small linear calcifications that were found in her left breast by mammography. No tumor was detected on palpation. The results of mammography, echography, and magnetic resonance imaging (Figure 1), suggested the presence of malignancy. On aspiration cytology, no cells were obtained. Breast-conservation surgery was performed. The tumor was macroscopically indistinct and had a diameter of 30 mm. The intraductal carcinoma was extended, and invasion to the surrounding tissue was not found (Figure 2). Small foci of linear calcification and necrosis were seen in the ducts that were occupied by tumor cells, and these exhibited a comedo pattern. There were no abundant secretory materials. In three-fourths of the lesions, the tumor cells had clear and abundant cytoplasm and compressed crescent-shaped or oval nuclei (Figure 3). The cytoplasm was vacuolated. Among these cells, some had atypical large nuclei with distinct nucleoli, and some had small round nuclei and modest eosinophilic cytoplasm-so-called "fried-egg cells" (Figure 4A). Each of the components presented different characteristics. The former were negative for periodic acid-Schiff stain (PAS), and the latter were positive. After treatment with diastase, the cells were negative for PAS (Figure 4B, C). Immunohistochemically, both types of cells were diffusely positive for cytokeratin (AE1/AE3) and focally positive for gross cystic disease fluid protein-15 (GCDFP-15), and were negative for vimentin, S-100 protein, smooth muscle actin and CD10. The carcinoma cells of both lipid-rich carcinoma and glycogen-rich carcinoma are immunohistochemically positive for estrogen (ER) and progesterone receptors, and negative for HER2. An ultrastructural study revealed lipid droplets in the cytoplasm of the vacuolated cells (Figure 1). However, glycogen particles were not shown. No nodal metastasis was found.
Lipid-rich carcinoma is a rare subtype of breast cancer that is histologically characterized by cells with numerous large and small vacuoles in their cytoplasm. The cells of lipid-rich carcinoma are divided into the following 3 forms: the histiocytoid type, the sebaceous type, and the type with apocrine extrusion of nuclei [3]. Two or 3 of these types often exist together, as in this case, where the histiocytoid and sebaceous types were observed. Fat staining of cryostat sections revealed the presence of a large amount of lipid within the cytoplasm. Lipid accumulation was also shown in an ultrastructural study. Nevertheless, the definition of lipid-rich carcinoma is controversial because it is unclear as to what percentage of vacuolated cells and which type of origin of the lipid vacuoles should confirm the diagnosis [4]. In our case, about 75% of the tumor cells had lipid-rich cytoplasm. Ramos and Taylor [5] have shown by electron microscopy that lipids originate from a secretory product of the neoplastic cells, and they do not consist of a degenerative material. They described the secretory vacuoles as being close to a markedly prominent Golgi apparatus, and the absence of autophagic vacuoles and the presence of prominent rough endoplasmic reticulum supported this speculation. Vera-Sempere and Llombart-Bosch [6] suggested a similar origin of the secretory product. However, Wrba et al. [7] reported no immunohistochemical or ultrastructural findings that could support the lipid secretion of carcinoma cells in their cases. Therefore, in their opinion, the term lipid-rich carcinoma, rather than lipid-secreting carcinoma, should be used, unless there is evidence of active lipid secretion.
Neoplasms of the breast that have clear cytoplasm occur in a variety of diseases, such as lipid-rich carcinoma, glycogen-rich carcinoma, apocrine carcinoma, secretory carcinoma, clearcell myoepithelioma, and clear-cell adenomyoepithelioma. In addition, soft-tissue neoplasms of the breast and chest wall, for example, clear-cell sarcoma, clear-cell rhabdomyosarcoma, and alveolar soft-part sarcoma, also need to be discriminated [8,9]. Skin tumors with clear cytoplasm must be distinguished too. Metastatic renal cancer of the clear-cell type of carcinoma has been reported, but they are highly rare [10]. In breast carcinomas, PAS stain and immunostaining are useful in the differential diagnosis. For example, glycogen-rich carcinoma and apocrine carcinoma are positive for PAS, and lipid-rich carcinoma is negative. Glycogen granules in glycogen-rich carcinoma become negative for PAS after diastase digestion; on the other hand, granular cytoplasm of apocrine carcinoma keeps positive for PAS after that treatment. Positive immunostain for the S-100 protein has been reported to be useful in the diagnosis of lipid-rich carcinoma [11], although in our case, immunostaining for this protein was negative; however, this is not useful in the differentiation between lipid-rich carcinoma and myoepithelial tumors. Immunostaining for smooth muscle actin, which is reactive in myoepithelial cells, is helpful in such cases. S-100 protein is frequently expressed in secretory carcinoma, too. But, most of secretory carcinomas are negative for ER. Soft-tissue tumors that have clear cytoplasm are ruled out by immunoreactivity for vimentin and negative stains for AE1/AE3 and GCDFP-15. GCDFP-15 has been reported to be useful in differentiating between primary breast carcinoma and metastatic carcinoma [12]. Metastatic clear cell renal cell carcinoma is positive for vimentin and CD10, and negative for GCDFP-15. In addition, GCDFP-15 is useful in the differential diagnoses among some primary breast carcinomas. Apocrine carcinoma indicates diffuse and intensive positive for GCDFP-15, but secretory carcinoma indicates negative for it.
A lesion with a combination of lipid-rich carcinoma and glycogen-rich carcinoma was reported by Kovacs and Krutsay [2]. In the present lesion, some of the carcinoma cells had glycogen in their cytoplasm. These cells had the same histological characteristic features as glycogen-rich carcinoma in that the cells had round nuclei at the center of pale eosinophilic cytoplasm and were positive for PAS and sensitive to diastase digestion. Glycogen, which can be demonstrated by electron microscopy [13], was not revealed in this case. This may have been because the electron microscopy was performed on paraffinembedded specimens. Both lipid-rich carcinoma and glycogen-rich carcinoma are the diagnoses that are determined by the metabolic products in the cytoplasm, and these are different from the diagnoses that are determined by the cellular types. However, diagnoses must be determined on the basis of not only examination of the intracytoplasmic products but also the histologic findings, because a varied amount of lipid and glycogen accumulation in cytoplasm was found in 30% and 85%, respectively, of mammary carcinomas [14,15]. Glycogen has also been demonstrated in mammary squamous cell carcinoma. Histologic findings of clear, foamy, or vacuolated cytoplasm are important for the diagnosis of lipid-rich carcinoma, and the findings of a fried-egg appearance are important for the diagnosis of glycogen-rich carcinoma.
Clinically, early detection of mammary lipid-rich carcinoma is presumed to be difficult. Except for a few reports that did not mention whether the carcinoma was invasive or intraductal, all reported cases of lipid-rich carcinoma were invasive. The ratio of intraductal carcinoma to invasive carcinoma in lipid-rich carcinoma was lower than that in ductal carcinoma. This is not only because of the low incidence of lipid-rich carcinoma but also because of the difficulty in detecting it. In the present case, the tumor was not palpable. Calcifications that were detected by medical examination led to the early discovery in this case.
Figures and Tables
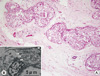 | Figure 3(A) Intraductal lipid-rich carcinoma. Neoplastic cells with vacuolated cytoplasms fill the mammary ducts. A comedo pattern with central necrosis is seen (H&E stain, ×100). (B) Electron microscopy showed lipid droplets (arrows) in the cytoplasm of tumor cells. |
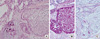 | Figure 4A component of glycogen-rich carcinoma (A, H&E stain, ×200; B, periodic acid-Schiff stain [PAS], ×200; C, PAS after diastase digestion, ×200). Cells with a small round nucleus and eosinophilic cytoplasm (A) are positive for periodic acid-Schiff stain (B), after treatment of diastase, negative for PAS (C). |
References
1. Aboumrad MH, Horn RC Jr, Fine G. Lipid-secreting mammary carcinoma. Report of a case associated with Paget's disease of the nipple. Cancer. 1963. 16:521–525.

2. Kovacs Z, Krutsay M. Lipid- and glycogen-rich carcinoma of the breast. Magy Onkol. 2002. 46:265–268.
3. van Bogaert LJ, Maldague P. Histologic variants of lipid-secreting carcinoma of the breast. Virchows Arch A Pathol Anat Histol. 1977. 375:345–353.

4. Umekita Y, Yoshida A, Sagara Y, Yoshida H. Lipid-secreting carcinoma of the breast: a case report and review of the literature. Breast Cancer. 1998. 5:171–173.

5. Ramos CV, Taylor HB. Lipid-rich carcinoma of the breast. A clinicopathologic analysis of 13 examples. Cancer. 1974. 33:812–819.

6. Vera-Sempere F, Llombart-Bosch A. Lipid-rich versus lipid-secreting carcinoma of the mammary gland. Pathol Res Pract. 1985. 180:553–558.

7. Wrba F, Ellinger A, Reiner G, Spona J, Holzner JH. Ultrastructural and immunohistochemical characteristics of lipid-rich carcinoma of the breast. Virchows Arch A Pathol Anat Histopathol. 1988. 413:381–385.

8. Govender D, Sabaratnam RM, Essa AS. Clear cell 'sugar' tumor of the breast: another extrapulmonary site and review of the literature. Am J Surg Pathol. 2002. 26:670–675.
9. Hanna NN, O'Donnell K, Wolfe GR. Alveolar soft part sarcoma metastatic to the breast. J Surg Oncol. 1996. 61:159–162.

10. Kannan V. Fine-needle aspiration of metastatic renal-cell carcinoma masquerading as primary breast carcinoma. Diagn Cytopathol. 1998. 18:343–345.

11. Varga Z, Robl C, Spycher M, Burger D, Caduff R. Metaplastic lipid-rich carcinoma of the breast. Pathol Int. 1998. 48:912–916.

12. Satoh F, Umemura S, Osamura RY. Immunohistochemical analysis of GCDFP-15 and GCDFP-24 in mammary and non-mammary tissue. Breast Cancer. 2000. 7:49–55.

13. Fujino S, Tezuka N, Sawai S, Konishi T, Inoue S, Kato H, et al. Glycogenrich clear cell carcinoma of the breast: a case report and review of the literature. Breast Cancer. 1996. 3:205–208.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download