Abstract
We describe a patient with breast cancer who relapsed with an extensive pulmonary lymphovascular tumor embolism. A 38-year-old female, who previously received neoadjuvant chemotherapy and curative resection of breast cancer, underwent adjuvant chemotherapy and was referred to the emergency room because of sudden-onset pleuritic chest pain lasting for 10 days. Despite a trial of empirical antibiotics, the chest pain and the extent of consolidative lung lesion on chest radiographs rapidly aggravated. We performed an open lung biopsy to confirm the etiology. The histopathological review revealed a hemorrhagic infarction caused by lymphovascular tumor emboli from a metastatic breast carcinoma. Palliative first-line chemotherapy was administered, consisting of ixabepilone and capecitabine, and the lung lesion improved markedly.
Pulmonary lymphovascular tumor embolism is a rare but important cause of rapidly progressing dyspnea in patients with cancer. A diagnosis of a pulmonary lymphovascular tumor embolism is rarely given before death, but the incidence of tumor involvement in the pulmonary lymphovascular structure is up to 26% in previous autopsy case reports [1,2]. Breast cancer is known as the most common primary source of microscopic pulmonary tumor emboli [3]. Previously reported cases of a pulmonary tumor embolism are mostly due to known primary malignancy, but in some cases, microscopic pulmonary emboli in a patient presenting with progressive dyspnea is the very first presentation of cancer [4]. We report a patient with breast cancer relapsed with an extensive pulmonary lymphovascular tumor embolism during adjuvant chemotherapy, which was diagnosed and treated successfully.
A 38-year-old female patient was diagnosed with clinical Stage IIIC (cT2cN3M0) infiltrating ductal carcinoma of the left breast with left supraclavicular lymph nodes metastases. She was treated with neoadjuvant chemotherapy consisting of docetaxel and doxorubicin. After six cycles of chemotherapy, the supraclavicular lymph node was not palpable with a partial response of the breast cancer and the axillary nodes. She underwent a curative resection for the breast cancer, and the final pathologic stage was ypStageIIIC (ypT2N3M0). Immunohistochemistry showed negative staining for the estrogen receptor (ER), progesterone receptor (PR), and human epithelial growth factor receptor 2 (HER2). Histological and nuclear grades were III/III and 3/3, respectively, and extensive endolymphatic tumor emboli were present. Adjuvant chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) was commenced, but on her visit for the third cycle she complained of sudden-onset right chest pain and cough. Her chest X-ray was within normal limits (Figure 1A). Ten days later, she felt right inspiratory chest pain and was referred to the emergency department. She complained of dyspnea on exertion (DOE), cough, and a mild febrile sense, however, she denied the high fever, sputum, pitting edema, or orthopnea. Her initial vital signs were a heart rate of 97 beats per minute, respiratory rate of 20 per minute, blood pressure of 133/68 mmHg, body temperature of 36.5℃, and oxygen saturation 97% on room air. Signs of respiratory distress were not definite. Fine crackles in the right upper lung field were present on chest auscultation but a pleural friction rub was not present. A chest X-ray demonstrated patchy consolidation on the right upper lung field (Figure 1B) and computed tomography (CT) showed right upper lobe (RUL) patchy consolidation with ill-defined small ground glass opacities (GGO) in the RUL and right lower lobe (RLL) (Figure 2A, B, respectively). The differential diagnosis from the radiological study was pneumonia with a fungal etiology but we could not rule out a bacterial etiology. The white blood cell count was 5,800/mm3, and the segmented neutrophil portion was 76.4%. The C-reactive protein level was 0.86 mg/dL (reference range, 0-0.5 mg/dL) and there was no evidence of pulmonary thromboembolism on chest CT.
We started empirical antibiotics with third generation cephalosporin and macrolide under the impression of community acquired pneumonia, but the follow-up chest CT at 1 week showed an increased extent of RUL patchy consolidation (Figures 1C, 2C, D). Furthermore, subjective symptoms of dyspnea and pleuritic chest pain rapidly aggravated. No microorganisms were identified on the initial respiratory specimen culture. Findings from chest X-rays and chest CT suggested fungal pneumonia, however, the patient had not experienced severe neutropenia for several months, and there were no predisposing clinical factors favoring a diagnosis of fungal pneumonia. We considered the probability of lymphangitic metastatic carcinoma or a fungal infection such as aspergillosis or mucormycosis. Therefore, we performed a video-assisted thoracoscopic lung biopsy for an accurate pathological diagnosis before administering antifungal agents. We thought it might be difficult to obtain a differential diagnosis using a percutaneous needle biopsy, because the nature of the lesion was infiltrative consolidation, not a mass.
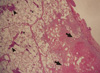
We performed a wedge resection of the RUL. The specimen was 6×3×3 cm, and a gross examination of the sections showed focal brownish discoloration of lung parenchyma without definite evidence of necrosis, tumor, hemorrhage, or a calcified area. A microscopic examination showed metastatic carcinoma with extensive endolymphatic tumor emboli and hemorrhagic infarctions (Figures 3, 4). In contrast, Gram staining revealed no microorganisms and Gomori's methenamine silver and periodic-acid Schiff stains revealed no fungal hyphae. The Mycobacterium tuberculosis-polymerase chain reaction result was also negative.
We confirmed that the patient was not under infection, so we discontinued antimicrobial agents, enrolled the patient to the clinical trial and started first-line palliative chemotherapy consisting of ixabepilone (Ixempra™; Bristol-Myers Squibb, New York, USA) and capecitabine (Xeloda®; Roche, Basel, Switzerland), as the disease had relapsed just 4 months after neoadjuvant docetaxel and doxorubicin and during adjuvant CMF chemotherapy (Figure 1D). This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A110448). After chemotherapy, the size of the consolidative lesion in the RUL and the extent of focal GGO in RUL and RLL decreased markedly. The pleuritic chest pain and DOE also improved. Currently, after 17 months, she is under palliative chemotherapy and regular follow-up with partial response to the disease (Figure 1E).
A pulmonary tumor embolism is defined as tumor cells within the pulmonary lymphovascular system that is not contiguous with metastatic foci [5]. The incidence of pulmonary tumor embolism varies widely among several studies, ranging from 3% to 26% [2]. Symptoms of microscopic pulmonary tumor emboli include clinically unexplained subacute, progressive dyspnea, hypoxia, chest pain, cough, and respiratory alkalosis [2,5,6]. Right-sided heart failure and pulmonary hypertension are occasionally noted [2].
A pulmonary tumor embolism is rarely diagnosed before death, because a histological diagnosis is rarely made and hard to distinguish from thromboembolism; according to one study, only 6% of patients were correctly diagnosed antemortem [1]. A ventilation-perfusion scan with peripheral, symmetric perfusion defects can be used for diagnosis, but its clinical benefit is limited due to low sensitivity [2,3]. Therefore, a histological or cytological diagnosis by open lung biopsy, transbronchial lung biopsy, or microvascular cytology drawn from a wedged pulmonary artery catheter is necessary [1,2]. In the present case, obstruction of pulmonary lymphatics and vasculature resulted in a peripheral lung infarction, and these presented as consolidation and GGO on chest radiography and CT. In contrast, the true extent of tumor involvement in the resected lung specimen was tiny emboli confined to the lymphatics or microvessels, which is much smaller than shown in imaging studies. The presence of consolidation and GGO on imaging studies makes the diagnosis more difficult, because excluding an infection (such as bacterial, viral or fungal pneumonia) is difficult in a clinical situation.
Pulmonary metastases with lymphovascular tumor emboli mimicking pneumonia were appropriately and timely diagnosed by wedge resection of the lung and improved after appropriate chemotherapy. The patient has currently survived more than 17 months after the event. Accurate and prompt diagnosis of a pulmonary tumor embolism and appropriate chemotherapy may provide an improved in the prognosis in these patients.
Standard treatment for a pulmonary tumor embolism is not established, except for systemic chemotherapy. The response to therapy and the prognosis largely depend on the nature of the primary malignancies. Some reports have indicated a good response to chemo-sensitive tumors, but the outcome of the disease is generally poor [1,4,7,8]. In a recent report, unlike the cancer cells at the primary site, those in the pulmonary lymphovasculature were enriched with stem cell-like markers (OCT3/4+, NANOG2+, CD44+/CD24-/low, ALDH1+), and these findings may explain the aggressiveness of the disease [8].
In patients with metastatic and locally advanced breast cancer resistant to both anthracyclines and taxanes, the superiority of capecitabine plus ixabepilone over capecitabine alone in terms of progression free survival and objective response has been demonstrated in a randomized phase III trial [9]. These clinical benefits were consistent across subgroups, including patients with ER-, PR-, and HER2-negative breast cancer, visceral metastases, more than two sites of metastatic disease, age ≥65 years, or those with HER2-positive breast cancer, which are traditionally associated with a poor prognosis [9,10]. In the present case, the ER-, PR-, and HER2-negative breast cancer relapsed soon after neoadjuvant chemotherapy with docetaxel plus doxorubicin (within 4 months) and presented as extensive pulmonary tumor emboli, which are usually life-threatening without prompt and appropriate treatment. We promptly started active chemotherapy with capecitabine plus ixabepilone and have achieved survival of more than 17 months by this time.
We recommend that if a patient with a known malignancy presents with a subacute course of progressive dyspnea with consolidation on imaging studies, the lung lesion does not improve with empirical antimicrobial treatment, and if the clinical setting is not compatible with infection or thromboembolism, the physician should consider the probability of pulmonary lymphovascular invasion with tumor emboli. Furthermore, the physician should make an effort to obtain pathological confirmation and should start chemotherapy as soon as possible.
Figures and Tables
Figure 1
(A) Chest PA at symptom presentation (10 days before emergency room visit). No remarkable findings are observed. (B) Chest PA at the emergency room. Patchy consolidative lesion on the right upper lobe (RUL) is shown (arrow). (C) After 1 week of empirical antibiotics for community acquired pneumonia, RUL consolidation had not improved, and consolidation near the interlobar fissure and hilum increased in extent (arrow and arrowhead). (D) After the open lung biopsy and just before palliative chemotherapy, the RUL and the right lower lobe (RLL) consolidative lesion (arrow) and ground glass opacity further increased. (E) After 17 months of palliative chemotherapy, consolidative lesions in RUL and RLL decreased markedly.
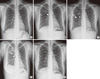
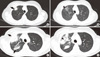
Figure 2
(A, B) Chest computed tomography (CT) scan at the emergency room. Patchy consolidative mass-like lesion in the right upper lobe (RUL) (arrow) with focal ground glass opacity (GGO) (arrowhead). (C, D) After 1 week of empirical antibiotics for community acquired pneumonia, RUL consolidation and consolidation near the interlobar fissure and hilum increased in extent (arrows), and GGO of the RUL increased in extent (arrowhead).
References
1. Goldhaber SZ, Dricker E, Buring JE, Eberlein K, Godleski JJ, Mayer RJ, et al. Clinical suspicion of autopsy-proven thrombotic and tumor pulmonary embolism in cancer patients. Am Heart J. 1987. 114:1432–1435.

2. Roberts KE, Hamele-Bena D, Saqi A, Stein CA, Cole RP. Pulmonary tumor embolism: a review of the literature. Am J Med. 2003. 115:228–232.

3. Sostman HD, Brown M, Toole A, Bobrow S, Gottschalk A. Perfusion scan in pulmonary vascular/lymphangitic carcinomatosis: the segmental contour pattern. AJR Am J Roentgenol. 1981. 137:1072–1074.

4. Gajdos C, Nierman DM, Moqtaderi FF, Brower ST, Lento P, Bleiweiss IJ. Microscopic pulmonary tumor emboli: an unusual presentation of breast cancer. Breast J. 2000. 6:273–275.

5. Bassiri AG, Haghighi B, Doyle RL, Berry GJ, Rizk NW. Pulmonary tumor embolism. Am J Respir Crit Care Med. 1997. 155:2089–2095.

6. Aiyappan V, Alwail A. Pulmonary tumor thromboembolism: a case report and review of literature. Ann Thorac Med. 2007. 2:169–170.

7. Ejlertsen B, Jensen MB, Rank F, Rasmussen BB, Christiansen P, Kroman N, et al. Population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. J Natl Cancer Inst. 2009. 101:729–735.

8. Kawasaki H, Ogura H, Arai Y, Baba S, Kosugi I, Tsutsui Y, et al. Aggressive progression of breast cancer with microscopic pulmonary emboli possessing a stem cell-like phenotype independent of its origin. Pathol Int. 2010. 60:228–234.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download