Abstract
Purpose
Idiopathic granulomatous mastitis (IGM) is a rare chronic inflammatory disease of unknown etiology. The diagnosis of IGM requires that other granulomatous lesions in the breast be excluded. Tuberculous mastitis (TM) is also an uncommon disease that is often difficult to differentiate from IGM. The purpose of this study is to develop a new algorithm for the differential diagnosis and treatment of IGM and TM.
Methods
Medical records of 68 patients (58 with IGM and 10 with TM) between July 1999 and February 2009 were retrospectively reviewed.
Results
The mean age of the patients was 33.5 (IGM) and 40 (TM) years (p=0.018). The median follow-up was 84 months. Of the total 10 patients with TM, 5 patients had a history of pulmonary tuberculosis. The most common symptoms of the diseases were breast lump and pain. However, axillary lymphadenopathy was more seen in TM (50%) compared to IGM (20.6%) (p=0.048). TM showed more cancer-mimicking findings on radiologic study (p=0.028). In IGM, 48 patients (82.7%) underwent surgical wide excision and 21 patients (36.2%) were managed with corticosteroid therapy and antibiotics. All of the TM patients received anti-tuberculosis medications and 9 patients (90%) underwent wide excision. The mean treatment duration was 2.8 months in IGM and 8.4 months in TM. Recurrence developed in 5 patients (8.6%) in IGM and 1 patient (10%) in TM.
Conclusion
This study shows different characteristics between IGM and TM. The IGM patients were younger and had more mastalgia symptoms than the TM patients. Axillary lymphadenopathy was seen more often in TM patients. Half of the TM patients had pulmonary tuberculosis or tuberculosis lymphadenitis. Surgical wide excision might be both therapeutic and useful for providing an exact diagnosis.
Idiopathic granulomatous mastitis (IGM) is a benign breast disease that was first described by Kessler and Wolloch in 1978 [1]. A rare inflammatory condition of the breast, it usually occurs in young parous women with a history of breast-feeding [1-6]. The most common clinical presentation is a firm, unilateral, and discrete breast mass that is often associated with inflammation of the overlying skin. In ≥50% of reported cases, the initial diagnosis was considered malignant or was suspected to be breast cancer. Other causes of mammary granuloma formation must be excluded before a confirmative diagnosis can be made, including sarcoidosis, Wegener's granulomatosis, tuberculosis, and a fungal infection because they require completely different treatments. Microbiological investigation is, therefore, very important. Cytological features can be difficult to distinguish from those of carcinoma and other granulomatous mastitis such as tuberculosis of the breast.
Tuberculous mastitis (TM) is an uncommon disease that is often difficult to differentiate from IGM and breast cancer when it presents as a lump. Breast tuberculosis should be considered a differential diagnosis in people with clinically suspicious breast lumps in high-risk populations or endemic areas. Use of corticosteroids and surgical wide excision of the lesions have been reported for the treatment of IGM. TM is an important differential diagnosis because of the implications of corticosteroid therapy.
In this study, we compared the clinicopathological features, management, and outcomes of IGM and TM in an effort to determine modalities for the differential diagnosis.
This study enrolled 68 IGM and TM patients diagnosed at one university hospital between July 1999 and February 2009. Of the patients, 58 patients had IGM and 10 patients had TM. After obtaining approval from the Institutional Review Board (AJIRB-MED-MDB-11-092), the following clinicopathological data were retrospectively collected from the medical records: patient demographics; pertinent history; clinical features; radiographic, histological, and microbiological results; treatment modalities; and follow-up recurrence data.
The histological diagnosis of granulomatous mastitis was made when a granulomatous reaction was centered on lobules and neither caseous necrosis nor any specific organisms was present. All slides were treated with hematoxylin and eosin and special stains such as Gram, Ziehl-Neelson stain for Mycobacterium tuberculosis, and periodic acid Schiff for fungal infections by one pathologist. Acid-fast bacillus (AFB) culture and polymerase chain reaction (PCR) for M. tuberculosis were also performed. PCR was performed using paraffin blocks.
Mean follow-up was 11.71 months and 21 months in IGM and TM, respectively.
Statistical analysis was performed using SPSS version 15.0 (SPSS Inc., Chicago, USA) by the biomedical informatics team. Differences between groups were evaluated using Pearson's χ2 test or Fisher's exact test. A p-value <0.05 was considered significant.
The patients were 17-65 years of age. The median age in the IGM and TM groups was 33.48 years and 40 years, respectively. Patients with IGM had significantly earlier onset than patients with TM (p=0.018). Most patients (97%) were of reproductive age. Six patients with IGM had a history of lactation history within 12 months prior to presentation and 1 patient with IGM had a history of oral contraceptive pill use (Table 1).
No patients with IGM had a history of connective tissue disease, sarcoidosis, tuberculosis, or other infectious granulomatous diseases. In contrast, 5 patients with TM (50%) had a history of pulmonary tuberculosis. The chief complaint was a breast lump and pain in both groups. There was no difference in the incidence of breast laterality. One patient with IGM presented with bilateral breast involvement.
The most common presenting symptoms of IGM were a breast mass (52 patients, 89.6%), mastalgia (49 patients, 84.4%), and overlying skin erythema (39 patients, 67.2%). In TM, a breast mass (7 patients, 70%), breast pain (5 patients, 50%), and axillary lymphadenopathy (5 patients, 50%) were noted. Significantly more patients with IGM complained of mastalgia (p=0.013). Axillary lymphadenopathy was more frequent in the patients with TM (p=0.048).
All 10 patients with TM and 57 patients with IGM underwent radiographic examination. Five patients with TM underwent both ultrasonography (US) and mammography (MMG). Five patients underwent US alone due to breast pain. Twenty-six patients with IGM underwent MMG and 57 underwent US. The one patient who did not undergo US already had an abscess drainage tube installed in other hospital. Radiologic findings were confirmed through cross-checking of 3 radiologists.
In patients with IGM, MMG revealed an asymmetric density with no distinct margins in 13 patients and an ill-defined mass in 7 patients. Axillary lymph node (LN) enlargement was detected using MMG in 2 patients with TM (Table 2). US examination showed a heterogeneous hypoechoic lesion in 26 patients with IGM and 3 patients with TM. An ill-defined hypoechoic mass was seen in 14 patients with IGM and in 5 patients with TM (Figure 1). Multifocal abscess cavities were seen in 16 patients with IGM. Axillary LN enlargement was present in 8 patients (80%) with TM. Clinical and radiological findings before biopsy suggested a suspicious malignant neoplasm in 7 patients with TM (70%) versus 17 patients with IGM (29.8%).
Fine-needle aspiration biopsy (FNAB) was performed in 24 patients with IGM and 6 patients with TM but was diagnostic in only 6 (25%) and 1 (16.6%), respectively. FNAB results were inconclusive in 18 (75%) patients with IGM and 5 (83.4%) patients with TM because of insufficient materials or nonspecific inflammatory findings. All women with an inconclusive FNAB underwent US-guided core biopsy or surgical excisional biopsy to obtain a definitive diagnosis. Twenty-three women had an US-guided core biopsy, of which 17 (73.9%) were diagnostic: 15 patients with IGM (75.0%) and 2 patients with TM (66.7%). The remaining 44 women underwent excisional biopsies that were subsequently confirmed the IGM and TM diagnoses.
In 58 patients, IGM was the final diagnosis and was characterized microscopically by the presence of lobulocentric non-necrotizing granulomas (clusters of epithelioid histiocytes) in which no microorganisms or features of other pathologic entities were identified (Figure 2). Additional microscopic findings included lymphocytes, plasma cells, neutrophils, and giant cells. The inflammation often extended into adjacent perilobular and interlobular tissues. Culture for alcohol AFB was done in patients with discharge. PCR was performed in 13 patients, including 9 with IGM and 4 with TM, the latter of which resulted in confirmative diagnoses. Except for 4 PCR-confirmed cases, 3 of 6 cases were AFB-positive in the breast tissue and 2 cases were AFB-positive in the enlarged axillary LN. One patient with TM in 2007 who was AFB-negative and treated with anti-tuberculosis medicine after clinical diagnosis was PCR-positive when the test was run again at the time of this writing.
Forty-five (77.6%) patients with IGM received antibiotics for the local infection at the time of presentation or preoperatively. The type, dosage, and duration of antibiotic treatment varied and were dependent on the degree of patient presentation and the culture results. All of the patients with TM received anti-tuberculous therapy comprising rifampicin (450 mg), isoniazid (400 mg), pyrazinamide (1,500 mg), and ethambutol (800 mg) for 2 months followed by rifampicin, isoniazid, and ethambutol for another 4-7 months. Extended treatment for 10-14 months was needed in 3 (30%) of the 10 patients, all of whom had discontinued drug therapy and had recurrent or persistent symptoms. Twenty-one patients (36.2%) with IGM received corticosteroid treatment. Prednisolone was administered at a dose of ≤30 mg and continued until complete remission was achieved, followed by a tapering schedule of usually 30, 20, 10, and 5 mg. Medication was administered once in the early morning for steroid hormones to match the physiologic diurnal rhythm. The mean duration of steroid treatment was 28 days (7-120 days).
Most of the patients in both groups underwent surgical management. Surgical procedures included incision and drainage and wide local excision. None of the patients who received incision and drainage experienced improved symptoms other than early symptom relief. Forty-eight patients with IGM (82.8%) and 9 patients with TM (90.0%) required surgical wide local excision. The mean treatment duration was 2.8 months (0.2-12 months) for patients with IGM and 8.4 months (1-14 months) for patients with TM. Compared with IGM, patients with TM had significantly longer treatment durations (p<0.001). Mean follow-up was 11.71 months and 21 months in patients with IGM and patients with TM, respectively.
Recurrence developed in 5 (8.6%) patients with IGM. The mean age was 34.2 years and the mean mass size was 2.5 cm. Breast pain developed 2-5 months after excision in 3 patients with IGM, all of whom were treated with steroid therapy. In the other 2 cases of recurrence, a painful breast lump appeared after steroid therapy and was successfully treated with wide local excision. Only one patient with TM experienced recurrence, which consisted of a mass lesion with axillary LN enlargement that appeared 22 months after the patient discontinued the anti-tuberculous therapy. The recurrence was successfully treated by restarting of the anti-tuberculous therapy.
IGM a rare benign breast disease [1,2]. Few articles exist in the literature, most of which are case reports and small series [7-10]. Most patients are young parous women, but males may also be affected [2,11]. The age at diagnosis is generally 20-50 years; however, patients 11 and 83 years of age were reported [4,12]. IGM commonly occurs in patients with a history of recent pregnancy and lactation [2,3]. This finding was reflected in our study (mean patient age, 33.48 years; range, 17-47 years). The etiology of IGM is not clear. Many agents, such as local irritants, viruses, mycotic, parasitic infections, hyperprolactinemia, diabetes mellitus, smoking, and alfa-1 antitrypsin deficiency, have been considered, but an autoimmune reaction is most favored [1,2,13,14]. Also, the observed similarity between IGM and autoimmune diseases such as granulomatous thyroiditis, granulomatous prostatitis, granulomatous orchitis, and response to steroid treatment is compatible with the autoimmune hypothesis [1]. Extravasated lactational secretions may elicit a local granulomatous response with lymphocyte and macrophage migration. Extravasations of luminal secretions may occur secondary to local trauma or infection causing damage to the ductal epithelium [2,4,15]. A chemical-induced reaction associated with use of oral contraceptive pills has also been suggested [5,16]. In our data, only 1 patient had previously used contraceptive pills, while 6 patients had lactated in the previous 12 months.
In the diagnosis of IGM, the differential diagnosis must include other granulomatous diseases such as TM, sarcoidosis, Wegener's granulomatosis, and breast cancer [1,15,17,18]. TM must be especially considered since it increases in endemic regions. In the western countries, TM is rare and is associated with 0.1% of all breast lesions. In developing countries like India, it constitutes approximately 3% of treatable breast conditions. Physicians must also be cautious when examining patients from high-risk populations or endemic areas. Recently, Bani-Hani et al. [16] stated that the largest reported series of IGM came from developing countries. As a result, they suggested that IGM might reflect TM underdiagnosis. The cytomorphologic pattern seen in TM is indistinguishable from that seen in IGM. Since it is not always possible to detect acid-fast bacilli in histological sections of TM, accurate diagnosis can safely be made only when additional clinical data are present. IGM and TM have similar clinical symptoms, including a solitary breast mass, chronic draining sinus tracts, or an abscess cavity [6,11,19]. A breast mass is the most common presentation in IGM. The mass will sometimes penetrate the breast skin or the underlying pectoralis muscle. Nipple retraction, sinus formation, and axillary lymphadenopathy may be seen in IGM patients [11,13,14,20]. These finding were also observed in TM and breast cancer. In this study, patients in both disease groups presented most commonly with a palpable breast mass, breast pain, and overlying erythema. IGM is usually unilateral, although bilateral involvement has been reported [2,15,21,22]. In this study, unilateral involvement of breast was seen at initial presentation and no difference was detected between the right and left breasts or breast quadrants in all patients but one.
Breast US and MMG in IGM are used primarily to rule out other breast diseases, especially breast carcinoma [16]. In this study, the MMG reports of 1 patient with TM and 4 patients with IGM were suspicious of breast carcinoma. Asymmetric diffusely increased density of the fibroglandular tissue was the most frequent nonspecific mammographic finding in our study. This finding can be seen in normal breast or other types of diseases, including cancer. On the other hand, no clear mammographic abnormalities were found in 5 patients. Most of the US evaluations revealed ill-defined, irregularly shaped areas of echogenicity. These findings are in keeping with reports in the literature [2,6,16]. Corresponding US of these 7 patients with TM showed various features: an irregular hypoechoic mass lesion in 5 cases, hypoechoic nodular structures in 3 cases, and enlarged axillary LN in 8 cases. These are also the most common US findings for breast carcinoma. For this reason, cytological and histological examinations are required in addition to imaging modalities for diagnostic accuracy.
Because clinical and imaging studies of IGM and TM are nonspecific, definitive diagnosis is made using histopathology [13]. Among the large series describing the FNAB features of IGM in the literature, the usefulness of FNAB in IGM has been debated, with some authors confirming the useful role of FNAB [23] and others concluding that the various causes of granulomatous inflammation cannot be confidently differentiated by FNAB [24]. But FNAB is still an option for tissue sampling because it is more easily available and provides faster results than core biopsy [24]. FNAB may also be helpful in differentiating malignancy from an inflammation. The cytopathology of TM reveals epithelioid histiocytes, Langhan's giant cells, granulomas, and caseous necrosis. Demonstration of AFB insections or positive culture for acid-fast bacilli confirms the diagnosis of TM. The presence of neutrophils is not common in TM. The pathological criteria for the diagnosis of IGM include granulomatous inflammation with multinucleated giant cells, epithelioid histiocytes, and occasional features of fat necrosis, abscesses, sinus tract, and eosinophils. It is centered on lobules, but extensive inflammation may obliterate this feature. Minor ductal and periductal inflammation is usually seen. A predominantly neutrophilic background and the absence of necrosis favor a diagnosis of IGM. It should be considered when high numbers of epithelioid histiocytes are seen. However, FNAB may not always differentiate between IGM and other granulomatous diseases of the breast, and a final diagnosis may require histological samples, negative microbiological investigations, and clinical correlations. In our study, we found US-guided core biopsy to be more accurate because they showed the tissue architecture. Core biopsy was diagnostic in 75% of IGM patients who underwent US-guided biopsy, whereas only 25% of the FNAB procedures were diagnostic. Common causes of FNAB failure included insufficient material and nonspecific findings (e.g., fat necrosis, abscess). The use of PCR in the diagnosis of TM is less often reported, mostly as a tool to distinguish TM from other forms of granulomatous mastitis in selected reports [25]. Our experience shows that PCR was performed in 4 patients of TM and all of these had confirmed diagnosis.
The optimal management of IGM remains controversial [4,11,26]. Treatment alternatives have been described in very few articles. In this study, surgical excision and antibiotics were the primary treatment modalities. There are limited data on the use of antibiotic therapy for the treatment of IGM in the literature [2]. However, many patients developed cellulitis, abscesses, and open draining sinuses. In these cases, initial empiric treatment with antibiotics should be administered. Cultures of all aspirate and biopsy samples should be obtained and can appropriately direct antimicrobial therapy. In this study, only 1 IGM sample was culture-positive for Staphylococcus epidermidis, while all of other IGM samples were culture-negative. One case of TM was culture-positive for Mycobacteria. Our experience shows that wide surgical excision was associated with a low recurrence. Surgical excision can be therapeutic as well as useful in providing an exact diagnosis. After excision, if there is no delayed wound healing, infection, or recurrence, further therapy is not needed for IGM. Limited surgical excisions may lead to recurrence [16]. Wide surgical excisions have been associated with lower recurrence and complication rates [2].
Corticosteroids comprise the treatment of choice as conservative management [27]. Satisfactory results have been reported with high doses of prednisone. Steroids have been used either after excision or before surgery. For complicated and resistant cases, steroids should be administered after excision. In 18 of our cases, we administered steroid therapy after surgery. Corticosteroid therapy of prednisolone 2-30 mg/day is recommended for at least 6 weeks and should be continued until complete remission [17]. In this study, 530 mg prednisolone was administered and continued until complete remission was achieved, followed by a tapering schedule. The mean duration of steroid treatment was 28 days. Corticosteroids may exacerbate infectious diseases of the breast; therefore, exclusion of an infectious etiology was essential before actual administration of steroid treatment in all of our cases. It is important that TM be fully resolved prior to steroid treatment, particularly in endemic areas. Furthermore, the side effects of steroid therapy include glucose intolerance and cushingoid features. None of our patients who received steroid therapy showed side effects. In the literature review, different recurrence rates (range, 5.5-50%) are reported after excision [4,14]. In this study, recurrence developed in 5 (8.6%) patients.
IGM and TM are rare inflammatory disease of the breast that tend to occur in young parous women. The disease process most commonly presents as a solitary breast mass. Breast carcinoma and other inflammatory diseases of the breast must be ruled out prior to treatment. IGM can be difficult to distinguish from TM by clinical symptoms and radiologic findings. Differential diagnosis is important because they require absolutely different treatments. This study shows clinical differences between IGM and TM: 1) Patients with IGM tended to be younger than TM (mean age, 33.5 and 40 years; p=0.018); 2) Axillary lymphadenopathy was seen more in TM than IGM (50% and 20.6%, p=0.048); 3) Mastalgia was more common in patients with IGM (84.4% and 50%, p=0.013); 4) In radiologic finding, TM was more cancer mimicking (p=0.028); 5) Half of the patients with TM had a history of pulmonary tuberculosis or tuberculosis lymphadenitis. For this reason, check the chest X-ray and pay close attention to a patient's medical history.
The diagnosis of IGM must be based on a multidisciplinary approach. A few of these cases were diagnosed clinically and radiologically before FNAB and core biopsy were performed, which emphasizes the recognition among surgeons, radiologists, and pathologists of this unusual but distinctive disorder. Increased recognition of these diseases will improve their understanding and management of them. Appropriate applications of surgical excision, steroid therapy, and combination with antibiotics if there is evidence of infection, are the most preferred treatment modality (Figure 3).
Figures and Tables
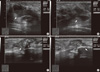 | Figure 1Ultrasonography of idiopathic granulomatous mastitis and tuberculous mastitis. (A) Idiopathic granulomatous mastitis: ill-defined hypoechoic mass with echogenic debridement. (B) Idiopathic granulomatous mastitis: finger-like projection. (C, D) Tuberculous mastitis: heterogeneous, hypoechoic masses with internal calcification. |
 | Figure 2Pathological characteristics of a patient with idiopathic granulomatous mastitis and tuberculous mastitis. (A) Idiopathic granulomatous mastitis: lobulocentric granulomas (H&E stain, ×200). Inlet: epithelioid granulomas and multinucleated giant cells were gathered around a glandular structure (H&E stain, ×400). (B) Tuberculous mastitis: biopsy specimen showing granulomatous inflammation with microabscess in the center (H&E stain,×200). Inlet: histopathological examination of the specimen revealed granulomas with caseous necrosis (H&E stain, ×400). (C) Tuberculous mastitis: acid-fast bacilli (Ziehl-Neelsen stain, ×1,000). |
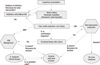 | Figure 3Treatment algorithm for the management of idiopathic granulomatous mastitis and tuberculous mastitis.
I&D=incision and drainage; IGM=idiopathic granulomatous mastitis; TM=tuberculous mastitis.
|
References
1. Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol. 1972. 58:642–646.

2. Akcan A, Akyildiz H, Deneme MA, Akgun H, Aritas Y. Granulomatous lobular mastitis: a complex diagnostic and therapeutic problem. World J Surg. 2006. 30:1403–1409.

3. Fletcher A, Magrath IM, Riddell RH, Talbot IC. Granulomatous mastitis: a report of seven cases. J Clin Pathol. 1982. 35:941–945.

4. Asoglu O, Ozmen V, Karanlik H, Tunaci M, Cabioglu N, Iqci A, et al. Feasibility of surgical management in patients with granulomatous mastitis. Breast J. 2005. 11:108–114.

5. Elsiddig KE, Khalil EA, Elhag IA, Elsafi ME, Suleiman GM, Elkhidir IM, et al. Granulomatous mammary disease: ten years' experience with fine needle aspiration cytology. Int J Tuberc Lung Dis. 2003. 7:365–369.
6. Han BK, Choe YH, Park JM, Moon WK, Ko YH, Yang JH, et al. Granulomatous mastitis: mammographic and sonographic appearances. AJR Am J Roentgenol. 1999. 173:317–320.

7. Dixon JM, Chetty U. Diagnosis and treatment of granulomatous mastitis. Br J Surg. 1995. 82:1143–1144.

9. Ayeva-Derman M, Perrotin F, Lefrancq T, Roy F, Lansac J, Body G. Idiopathic granulomatous mastitis: review of the literature illustrated by 4 cases. J Gynecol Obstet Biol Reprod (Paris). 1999. 28:800–807.
10. Belaabidia B, Essadki O, el Monsouri A, Squalli S. Idiopathic granulomatous mastitis: apropos of 8 cases and review of the literature. Gynecol Obstet Fertil. 2002. 30:383–389.
11. Erhan Y, Veral A, Kara E, Ozdemir N, Kapkac M, Ozdedeli E, et al. A clinicopathologic study of a rare clinical entity mimicking breast carcinoma: idiopathic granulomatous mastitis. Breast. 2000. 9:52–56.

12. Lai EC, Chan WC, Ma TK, Tang AP, Poon CS, Leong HT. The role of conservative treatment in idiopathic granulomatous mastitis. Breast J. 2005. 11:454–456.

13. Imoto S, Kitaya T, Kodama T, Hasebe T, Mukai K. Idiopathic granulomatous mastitis: case report and review of the literature. Jpn J Clin Oncol. 1997. 27:274–277.

14. Schelfout K, Tjalma WA, Cooremans ID, Coeman DC, Colpaert CG, Buytaert PM. Observations of an idiopathic granulomatous mastitis. Eur J Obstet Gynecol Reprod Biol. 2001. 97:260–262.

15. Diesing D, Axt-Fliedner R, Hornung D, Weiss JM, Diedrich K, Friedrich M. Granulomatous mastitis. Arch Gynecol Obstet. 2004. 269:233–236.

16. Bani-Hani KE, Yaghan RJ, Matalka II, Shatnawi NJ. Idiopathic granulomatous mastitis: time to avoid unnecessary mastectomies. Breast J. 2004. 10:318–322.

17. Jorgensen MB, Nielsen DM. Diagnosis and treatment of granulomatous mastitis. Am J Med. 1992. 93:97–101.

18. Donn W, Rebbeck P, Wilson C, Gilks CB. Idiopathic granulomatous mastitis: a report of three cases and review of the literature. Arch Pathol Lab Med. 1994. 118:822–825.
19. Pouchot J, Foucher E, Lino M, Barge J, Vinceneux P. Granulomatous mastitis: an uncommon cause of breast abscess. Arch Intern Med. 2001. 161:611–612.

20. Sakurai T, Oura S, Tanino H, Yoshimasu T, Kokawa Y, Kinoshita T, et al. A case of granulomatous mastitis mimicking breast carcinoma. Breast Cancer. 2002. 9:265–268.

21. Yip CP, Jayaram G, Swain M. The value of cytology in granulomatous mastitis: a report of 16 cases from Malaysia. Aust N Z J Surg. 2000. 70:103–105.

22. Heer R, Shrimankar J, Griffith CD. Granulomatous mastitis can mimic breast cancer on clinical, radiological or cytological examination: a cautionary tale. Breast. 2003. 12:283–286.

24. Martínez-Parra D, Nevado-Santos M, Meléndez-Guerrero B, García-Solano J, Hierro-Guilmain CC, Pérez-Guillermo M. Utility of fine-needle aspiration in the diagnosis of granulomatous lesions of the breast. Diagn Cytopathol. 1997. 17:108–114.

25. Tse GM, Poon CS, Ramachandram K, Ma TK, Pang LM, Law BK, et al. Granulomatous mastitis: a clinicopathological review of 26 cases. Pathology. 2004. 36:254–257.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download