1. Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981. 48:384–394.

2. Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004. 22:3608–3617.

3. Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983. 52:2349–2354.

4. Lai R, Dang CT, Malkin MG, Abrey LE. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer. 2004. 101:810–816.

5. Lower EE, Drosick DR, Blau R, Brennan L, Danneman W, Hawley DK. Increased rate of brain metastasis with trastuzumab therapy not associated with impaired survival. Clin Breast Cancer. 2003. 4:114–119.

6. Clayton AJ, Danson S, Jolly S, Ryder WD, Burt PA, Stewart AL, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004. 91:639–643.

7. Ono M, Ando M, Yunokawa M, Nakano E, Yonemori K, Matsumoto K, et al. Brain metastases in patients who receive trastuzumab-containing chemotehrapy for HER2-overexpressing metastatic breast cancer. Int J Clin Oncol. 2009. 14:48–52.

8. Perou CM, Sorlie T, Eisen MB, Van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000. 406:747–752.

9. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001. 98:10869–10874.

10. Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003. 100:8418–8423.

11. Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005. 11:5678–5685.

12. Smid M, Wang Y, Klijn JG, Sieuwerts AM, Zhang Y, Atkins D, et al. Genes associated with breast cancer metastatic to bone. J Clin Oncol. 2006. 24:2261–2267.

13. Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003. 3:537–549.

14. Deckers M, van Dinther M, Buijs J, Que I, Lowik C, Van der Pluijm G, et al. The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res. 2006. 66:2202–2209.

15. Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005. 436:518–524.

16. Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008. 68:3108–3114.

17. Slimane K, Andre F, Delaloge S, Dunant A, Perez A, Grenier J, et al. Risk factors for brain relapse in patients with metastatic breast cancer. Ann Oncol. 2004. 15:1640–1644.

18. Kaal EC, Vecht CJ. CNS complications of breast cancer: current and emerging treatment options. CNS Drugs. 2007. 21:559–579.
19. Graesslin O, Abdulkarim BS, Coutant C, Huguet F, Gabos Z, Hsu L, et al. Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol. 2010. 28:2032–2037.

20. Gonzalez-Angulo AM, Cristofanilli M, Strom EA, Buzdar AU, Kau SW, Broglio KR, et al. Central nervous system metastases in patients with high-risk breast carcinoma after multimodality treatment. Cancer. 2004. 101:1760–1766.

21. American Joint Committee on Cancer (AJCC). AJCC Cancer Staging Manual. 2010. 7th ed. New York: Springer.
22. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005. 365:1687–1717.
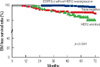
23. Lee SS, Ahn JH, Kim MK, Sym SJ, Gong G, Ahn SD, et al. Brain metastases in breast cancer: prognostic factors and management. Breast Cancer Res Treat. 2008. 111:523–530.

24. Korean Breast Cancer Society. 2006-2008 Breast Cancer Facts & Figures. 2008. Seoul: Korean Breast Cancer Society;5–12.









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download