Abstract
Purpose
B-cell lymphoma (bcl)-2 is an anti-apoptotic gene, and it is a poor prognostic factor in various malignant tumors. However, the prognostic significance of bcl-2 expression in breast cancer remains controversial. We investigated the prognostic significance of bcl-2 according to cancer molecular subtype.
Methods
We analyzed 411 patients with primary invasive breast cancer who underwent surgery at our institution between 1999 and 2001. The subtypes were classified as luminal (estrogen receptor [ER]+ and/or progesterone receptor [PR]+, irrespective of human epidermal factor receptor 2 [HER2]), triple-negative (ER-, PR-, and HER2-), or HER2 (ER- ,PR-, and HER2+).
Results
A total of 236 (57.4%) cases were positive for bcl-2, and bcl-2 expression was significantly associated with earlier stage, lower grade, expression of hormone receptor positivity, and HER2 negativity. No difference in disease-free survival (DFS) was observed based on bcl-2 expression. However, the prognostic significance of bcl-2 varied with subtype; bcl-2 was not a prognosticator in patients with the luminal and HER2 subtypes. However, patients with bcl-2(+) tumors of the triple-negative subtype showed significantly worse DFS than those with bcl-2(-) tumors (p=0.048). In a multivariate analysis, bcl-2 expression remained a significant predictor of recurrence in patients with the triple-negative subtype (hazard ratio, 3.26; 95% confidence interval, 1.40-7.59; p=0.006).
Figures and Tables
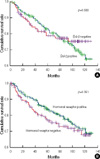 | Figure 1Disease-free survival according to bcl-2 expression (A) and disease free survival according to hormonal receptor expression (B). |
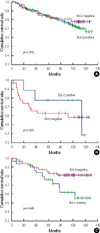 | Figure 2Disease-free survival according to bcl-2 expression in hormonal receptor positive breast cancer (A), in HER2 positive breast cancer (B), in triple negative breast cancer (C). |
Table 3
Clinicopathologic characteristic of triple negative breast cancer patients according to bcl-2 expression

References
1. Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003. 100:8418–8423.

2. Zhou T, Yang L, Ma GM, Li CX, Bai Y, Zhao JA, et al. Clinicopathologic features and prognosis of triple negative breast cancer. Zhonghua Yi Xue Za Zhi. 2009. 89:2261–2264.
3. Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer-current status and future directions. Ann Oncol. 2009. 20:1913–1927.

4. Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005. 106:408–418.

5. Lu PJ, Lu QL, Rughetti A, Taylor-Papadimitriou J. Bcl-2 overexpression inhibits cell death and promotes the morphogenesis, but not tumorigenesis of human mammary epithelial cells. J Cell Biol. 1995. 129:1363–1389.
6. McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LW, Hsieh JT, et al. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992. 52:6940–6944.
7. Pietenpol JA, Papadopoulos N, Markowitz S, Willson JK, Kinzler KW, Vogelstein B. Paradoxical inhibition of solid tumor cell growth by bcl2. Cancer Res. 1994. 54:3714–3717.
8. Chen MK, Yang SF, Lai JC, Yeh KT, Yang JS, Chen LS, et al. Expression of bcl-2 correlates with poor prognosis and modulates migration of nasopharyngeal carcinoma cells. Clin Chim Acta. 2010. 411:400–405.

9. O'Brien S, Moore JO, Boyd TE, Larratt LM, Skotnicki AB, Koziner B, et al. 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. J Clin Oncol. 2009. 27:5208–5212.
10. Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009. 15:1126–1132.

11. Kroger N, Milde-Langosch K, Riethdorf S, Schmoor C, Schumacher M, Zander AR, et al. Prognostic and predictive effects of immunohistochemical factors in high-risk primary breast cancer patients. Clin Cancer Res. 2006. 12:159–168.

12. Zhang GJ, Tsuda H, Adachi I, Fukutomi T, Yamamoto H, Hirohashi S. Prognostic indicators for breast cancer patients with one to three regional lymph node metastases with special reference to alterations in expression levels of bcl-2, p53 and c-erbB-2 proteins. Jpn J Clin Oncol. 1997. 27:371–377.

13. Elledge RM, Green S, Howes L, Clark GM, Berardo M, Allred DC, et al. Bcl-2, p53, and response to tamoxifen in estrogen receptor-positive metastatic breast cancer: a Southwest Oncology Group study. J Clin Oncol. 1997. 15:1916–1922.

14. Hellemans P, van Dam PA, Weyler J, van Oosterom AT, Buytaert P, Van Marck E. Prognostic value of bcl-2 expression in invasive breast cancer. Br J Cancer. 1995. 72:354–360.

15. Gurova KV, Kwek SS, Koman IE, Komarov AP, Kandel E, Nikiforov MA, et al. Apoptosis inhibitor as a suppressor of tumor progression: expression of Bcl-2 eliminates selective advantages for p53-deficient cells in the tumor. Cancer Biol Ther. 2002. 1:39–44.

16. Lee KH, Im SA, Oh DY, Lee SH, Chie EK, Han W, et al. Prognostic significance of bcl-2 expression in stage III breast cancer patients who had received doxorubicin and cyclophosphamide followed by paclitaxel as adjuvant chemotherapy. BMC Cancer. 2007. 7:63.

17. Lee JH, Lee ES, Kim CH. Expression of bcl-2 and apoptosis and its relationship to clinicopathological prognostic factors in breast cancer: a study with long term follow-up. J Korean Breast Cancer Soc. 2004. 7:92–97.

18. Sjostrom J, Krajewski S, Franssila K, Niskanen E, Wasenius VM, Nordling S, et al. A multivariate analysis of tumour biological factors predicting response to cytotoxic treatment in advanced breast cancer. Br J Cancer. 1998. 78:812–815.

19. Kim K, Chie EK, Han W, Noh DY, Park IA, Oh DY, et al. Prognostic value of p53 and bcl-2 expression in patients treated with breast conservative therapy. J Korean Med Sci. 2010. 25:235–239.

20. Chen HH, Su WC, Guo HR, Chang TW, Lee WY. p53 and c-erbB-2 but not bcl-2 are predictive of metastasis-free survival in breast cancer patients receiving post-mastectomy adjuvant radiotherapy in Taiwan. Jpn J Clin Oncol. 2002. 32:332–339.

21. Choi NK, Kim SY, Kim TY, Chae MK, Baek MJ, Lim CW, et al. Clinical correlation of c-erbB-2, p53, bcl-2, and c-myc expression in patients with breast cancer. J Korean Breast Cancer Soc. 2002. 5:125–134.

22. Hong YK, Baik SS, Chung MS, Yoon HS. Expression of cyclin D1 and bcl-2 in infiltrative ductal carcinoma of the breast: their correlations and clinical implications. J Breast Cancer. 2008. 11:172–179.

23. Costa SD, Lange S, Klinga K, Merkle E, Kaufmann M. Factors influencing the prognostic role of oestrogen and progesterone receptor levels in breast cancer--results of the analysis of 670 patients with 11 years of follow-up. Eur J Cancer. 2002. 38:1329–1334.

24. Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008. 26:2373–2378.

25. Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006. 24:4738–4745.

26. Marcucci G, Moser B, Blum W, Stock W, Wetzler M, Kolitz JE, et al. A phase III randomized trial of intensive induction and consolidation chemotherapy (+/-) oblimersen, a pro-apoptotic Bcl-2 antisense oligonucleotide in untreated acute myeloid leukemia patients >60 years old. 2007 ASCO Annual Meeting. 2007. 25. Abstract #7012.

27. Manero F, Gautier F, Gallenne T, Cauquil N, Grée D, Cartron PF, et al. The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res. 2006. 66:2757–2764.





 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download