Abstract
Purpose
Adipocytokines, such as leptin, resistin, and adiponectin, are associated with obesity and breast cancer. Several studies have indicated that adipocytokines may influence tumor growth or differentiation. The aims of this study were to determine the expression of leptin, leptin receptor (ObR), adiponectin and adiponectin receptor (AdipoR) in human breast cancer, to evaluate their prognostic significance in the breast cancer.
Methods
Specimens
from 198 patients with primary breast cancer were enrolled, and representative paraffin tumor blocks were selected for constructing tissue microarrarys (TMA). Immunohistochemical staining for leptin, ObR, adiponectin, and AdipoR was performed using TMA, and the clinicopathologic characteristics were evaluated from the patient's medical records.
Results
Stage 0 breast cancer accounted for 41 cases, and 157 cases were invasive cancer. Positive rates of leptin and ObR expression in the ductal carcinoma in situ (DCIS) group were significantly higher than those of the invasive cancer group (97.4% vs. 34.0%, p<0.001; 74.4% vs. 29.8%, p<0.001). However, positive rates of adiponectin and AdipoR expression in the invasive cancer group were significantly higher than those in the DCIS group (53.7% vs. 33.3%, p=0.024; 59.9% vs. 26.3%, p<0.001). High leptin expression was significantly associated with high Ki-67 expression (p=0.016). High adiponectin expression was significantly correlated with smaller tumor size (p=0.001).
The relationship between breast cancer and obesity has been recognized for many years [1,2]. The postulated mechanisms for the increased risk of breast cancer in obese women are elevated estrogen levels and insulin resistance and the influence of insulin-like growth factor (IGF) in the pathogenesis of breast cancer [3,4]. Multiple studies have indicated that some adipose tissue-derived hormones called adipocytokines, such as leptin and adiponectin, may significantly influence the growth and proliferation of tumors [5-7].
Leptin, a product of the obese (Ob) gene, is produced predominantly in adipose tissue and expressed in normal mammary epithelial cells and malignant breast tissues [8-10]. Some investigators have reported that expression of leptin and leptin receptor (ObR) is significantly higher in breast carcinoma compared to normal mammary tissue [11,12]. Leptin is involved in a variety of functions including appetite regulation, bone formation, reproduction, and angiogenesis [13]. Several studies have investigated the effects of leptin on breast cancer, and suggest that leptin may affect processes related to cancer initiation and progression, resulting in metastatic development [9,11,14,15].
Leptin acts through its receptor (ObR), which is encoded by the Ob gene. In human tissues, four different ObR variants have been described but only the long ObR isoform (ObR1) has full signaling potential [16]. Binding of leptin to ObR activates the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway, and induction of JAK stimulates phosphoinositol-3-kinase (PI3Kinase). Activation of PI3Kinase can increase cell migration and invasion via the Rac/Rho pathways, and stimulate the major growth and survival Akt pathway [7].
Adiponectin is another adipocytokine predominantly secreted by adipocytes [6]. In contrast to other adipocytokines, adiponectin levels are inversely correlated with central fat accumulation [17], and an inverse correlation was found between plasma adiponectin levels and the histological grade of breast cancer [18,19]. However, the precise effects of adiponectin in breast cancer are still unclear.
Two types of adiponectin receptors (AdipoR1 and R2) have been described, and adiponectin acts via those receptors. AdipoR1 acts mainly through adenosine monophosphate-activated protein kinase (AMPK) pathways, whereas AdipoR2 is more closely linked to the activation of the peroxisome proliferator-activated receptor alpha pathway. The growth inhibitory effect of adiponectin is influenced mainly through AMPK activation, while anti-cancer effects of adiponectin are mainly achieved by activation of AdipoR1 [20,21].
Leptin stimulates the invasion of breast cancer cells [11,14,22]. However, the relationship between leptin, ObR, adiponectin, AdipoR, and breast cancer invasiveness has not been investigated in human tissue. In the present study, we monitored the expression of leptin, ObR, adiponectin and AdipoR in breast cancer specimens using immunohistochemistry and analyzed how changes in expression correspond to clinicopathological parameters including breast cancer invasiveness.
A total of 198 patients with primary breast cancer who underwent surgery between January 2003 and April 2008 at Daegu Catholic University Medical Center were included. All resected specimens were stained with hematoxylin and eosin (H&E) and histologically examined. According to the pathologic diagnosis, 41 patients had ductal carcinoma in situ (DCIS), and 157 patients had invasive breast cancer.
The clinicopathological characteristics such as menopausal state, body mass index (BMI), and tumor invasiveness were evaluated based on pathological reports and medical records. Prognostic factors including tumor size, nodal status, distant metastasis, histological grade, lymphovascular invasion, and estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER2), Bcl-2, Ki67, and p53 expression were evaluated in invasive breast cancer. Lesion staging was assessed according to the sixth edition of the American Joint Committee on Cancer (AJCC) staging manual for breast cancer.
Representative paraffin tumor blocks were selected according to the primary evaluation of H&E-stained slides before they were prepared for TMA. Two tumor tissue cores (1 mm in diameter) were taken from each of the donor breast cancer tissue blocks with a manual punch arrayer (Quick-Ray™; Uni-Tech Science, Seoul, Korea). The cores were placed in a new recipient paraffin block that ultimately contained 72-96 tissue cores. Each array block contained both tumor and control tissue samples. Multiple sections (5-µm in thickness) were cut from the TMA blocks and then mounted onto microscope slides. The TMA H&E-stained sections were reviewed under light microscopy to confirm the presence of representative tumor areas.
Immunohistochemistry was conducted on 5 µm-thick TMA tissue sections using the Bond Polymer Intense Detection System (Leica Microsystems, Victoria, Australia) according to the manufacturer's instruction with minor modifications. Briefly, the 5 µm-thick sections of formalin-fixed and paraffin-embedded TMA tissues were deparaffinized with Bond Dewax Solution (Leica Microsystems), and an antigen retrieval procedure was performed using Bond ER Solution (Leica Microsystems) for 30 minutes at 100℃. The endogenous peroxidase was quenched by a 5-minute incubation with hydrogen peroxide. Sections were incubated for 15 minutes at ambient temperature with a rabbit polyclonal anti-leptin antibody (ab16227, 1:150; Abcam, Cambridge, UK), a rabbit polyclonal anti-leptin receptor antibody (ab60042, 1:150; Abcam), a mouse monoclonal anti-adiponectin antibody (ab22554, 1:150; Abcam) and a goat polyclonal anti-adiponectin receptor antibody (ab77611, 1:200; Abcam) using a biotin-free polymeric horseradish peroxidase-linker antibody conjugate system in a Bond-Max automatic slide stainer (Leica Microsystems). Human adipocytes (leptin and adiponectin), heptocellular carcinoma (leptin receptor) and normal liver (adiponectin receptor) tissues were used as positive controls.
Leptin, ObR, adiponectin, and AdipoR expression levels were graded on a scale of 0 to 2 based on staining intensity and proportion of positive tumor cells by an expert pathologist who was blinded to the patient's clinical records. The staining was scored as 0 if no cancer cells were reactive, 1 if staining was weakly positive in <2/3 of cancer cells, or strongly positive in <1/3 of cancer cells, and 2 if staining was weakly positive in >2/3 of cancer cells, or strongly positive in >1/3 of cancer cells. Immunohistochemical staining in breast cancer tissue was classified as negative (score 0) or positive (score 1 and 2).
A cutoff value of 10% for the stained nuclei was used to define ER and PR positivity. Cytoplasmic staining with any intensity in >10% of the tumor cells was scored as positive for Bcl-2. Membranous staining for HER2 with strong complete staining in 10% of the tumor cells was regarded as HER2 overexpression. The p53 staining was scored positive if >10% of the cells were stained with strong intensity. The Ki-67 labeling index was expressed as a percentage and was graded as "high" if the number of positive cells was ≥10%.
The statistical analysis was performed using SPSS version 15.0 (SPSS Inc., Chicago, USA). The relationship between leptin, ObR, adiponectin, AdipoR expression and clinicopathological features was assessed by the chi-square test. A p-value of less than 0.05 was considered statistically significant.
The average age of the 198 patients with breast cancer was 50.87±11.10 years (range, 25-81 years). Among 197 female patients, 95 (47.3%) were postmenopausal and 106 (52.7%) were premenopausal. The mean BMI was 24.1±3.4 kg/m2. According to criteria of the World Health Organization, a BMI ≥25 kg/m2 is defined as overweight, a BMI between 18.5 kg/m2 and 24.9 kg/m2 is normal, and a BMI <18.5 kg/m2 is considered underweight. A total of 69 patients (36.7%) were overweight, and 119 patients (63.3%) were normal or underweight (Table 1). TNM staging was as follows: 41 patients (20.7%) had stage 0, 57 patients (28.8%) had stage I, 71 patients (35.9%) had stage II, 26 patients (13.1%) had stage III, and three (1.5%) patients had stage IV. The histological types of DCIS were comedo (n= 16), solid (n=10), papillary (n=11), and cribriform (n=4), and those of invasive cancers were ductal carcinoma not otherwise specified (n=154), lobular carcinoma (n=1), medullary carcinoma (n=1), and metaplastic carcinoma (n=1).
We constructed two DCIS TMAs and four invasive breast cancer TMAs, which included specimens from 41 DCIS and 157 invasive breast cancer cases. Some of the tissue specimens that were partly lost during TMA construction or were unavailable were excluded. Figure 1 shows representative micro-photograph of immunohistochemical expression of the adipocytokines and their receptors. Positive expression rates for leptin, ObR, adiponectin, and AdipoR were 47.1% (89/189), 38.9% (74/190), 49.5% (93/188), and 53.2% (101/190), respectively.
As shown in Figure 2, positive rates of leptin and ObR expression in the DCIS group were significantly higher than those of the invasive cancer group (97.4% vs. 34.0%, p<0.001; 74.4% vs. 29.8%, p<0.001), and positive rates of adiponectin and AdipoR expression in the invasive cancer group were significantly higher than those of the DCIS group (53.7% vs. 33.3%, p=0.024; 59.9% vs. 26.3%, p<0.001).
Comparing the expression of leptin, ObR, adiponectin, and AdipoR with menopausal status and BMI, no significant correlation was observed and a comparison according to the histological subtypes of DCIS and invasive breast cancer showed no significant differences in the expression levels of each of the adipocytokines and their receptors (Table 2).
We analyzed the correlation between leptin, ObR, adiponectin, AdipoR expression and prognostic factors in invasive breast cancer. The results showed that leptin expression was significantly associated with high Ki-67 expression (p=0.016), and ObR expression was significantly associated with negative Bcl-2 expression (p=0.007). However, neither leptin expression nor ObR expression was significantly correlated with menopausal state, BMI, distant metastases, histological grade, HER2 expression, or p53 expression (Table 3).
Adiponectin expression was significantly associated with lower T-stage (p=0.028) in invasive breast cancer, but not with menopausal state, BMI, N stage, distant metastases, histological grade, lymphovascular invasion, hormonal receptor status, HER2 expression, or the other immunohistochemical parameters. AdipoR expression was significantly associated with high Ki-67 expression (p=0.029) in invasive breast cancer (Table 4).
Leptin expression was significantly associated with ObR expression (p<0.001), and adiponectin expression was significantly associated with AdipoR expression (p<0.001). However, leptin and adiponectin expression were not significantly correlated (p=0.463), and neither was ObR or AdipoR (p=0.175).
We observed that leptin, ObR, adiponectin, and AdipoR were significantly related to invasiveness of breast cancers. Positive rates of leptin and leptin receptor expression observed in the DCIS group were higher than those of the invasive cancer group, but positive rates of adiponectin and AdipoR expression were higher in the invasive cancer group. These results suggest that losses of leptin and ObR expression could be associated with breast cancer invasion. We assume that leptin and ObR expression is related to an early stage of carcinogenesis such as cancer development, while adiponectin and AdipoR may be associated with invasiveness of breast cancer. Our results also showed that leptin expression was significantly associated with a high Ki-67 labeling index, suggesting that leptin is associated with the proliferation of breast cancer cells, which is supported by recent studies [5-7,14].
Although some in vitro studies have demonstrated that leptin promotes invasiveness of human breast cancer cells, epidemiological studies showing a relation between leptin expression and invasiveness of breast cancer have not been reported. Saxena et al. [14] showed that bidirectional crosstalk between leptin and IGF-1 signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptors. McMurtry et al. [22] showed that leptin increases the invasiveness and matrix metallopeptidase 2 activity of breast cancer cells, which are mediated by Jun N-terminal kinases activation. In contrast, our results revealed that leptin and ObR expression was inversely correlated with breast cancer invasiveness. Additionally, leptin and ObR expression was not associated with distant metastasis, histological grade, lymphovascular invasion, or the other immunohistochemical parameters. From these results, we suggest that leptin and ObR are related to tumorigenesis of breast cancer, but not invasiveness.
A number of studies were initiated to understand the mechanisms associated with leptin and mammary tumor development [23,24]. Adipocytokines circulating in the blood exert their biological actions on target cells not only by classical endocrine mechanisms but also through paracrine or autocrine pathways [23-25]. Expression of leptin in breast cancer tissue is not representative of serum leptin concentrations, and is considered to be a product of the paracrine pathway [23]. Leptin mediates the estrogenic stimulation of tumor cells through a paracrine mechanism, and increases other factors that contribute to cell proliferation and angiogenesis during mammary tumor growth [24]. Furthermore, the autoregulation of leptin augments its signal by stimulating expression of itself and its receptor which supports an autocrine function [25]. Our results showed that leptin expression was significantly associated with ObR expression, and that adiponectin expression was significantly associated with AdipoR expression. Furthermore, leptin and adiponectin appear to have antagonistic effects in breast cancer invasion, although they were not significantly correlated. We suppose that leptin and adiponectin autoregulate their receptor through an autocrine pathway, and that leptin and adiponectin affect each other through a paracrine pathway.
The precise effects of adiponectin on breast cancer risk are still unclear. Epidemiological studies have reported a significant inverse association between adiponectin and breast cancer risk [17,19], and several in vitro studies have demonstrated a growth inhibitory action of adiponectin on breast cancer cells [26,27]. In contrast, Karaduman et al. [28] reported that tissue adiponectin levels in patients with breast cancer were significantly higher than healthy individuals, and that high tissue adiponectin levels were associated with a significantly increased risk for breast cancer compared with those with low tissue adiponectin levels.
The relationship between adiponectin expression and breast cancer invasiveness is still unknown. Pfeiler et al. [29] reported a positive correlation between lymphovascular and vascular invasion and AdipoR2 but not AdipoR1 expression. Jardé et al. [10] showed that adiponectin expression is higher in normal adjacent tissue than in neoplastic tissue, and that adiponectin expression in invasive ductal carcinoma is higher than that of in situ ductal carcinoma. In our study, we found that adiponectin and AdipoR expression in invasive breast cancer was significantly higher than that of DCIS, suggesting that adiponectin and AdipoR are related to breast cancer invasiveness. In this way, adiponectin may be associated with the progression and invasiveness of tumors and involved in later stages of breast cancer development.
As obesity is a key factor associated with circulating adipocytokine levels, obesity indices such as BMI may be related to adipocytokines and their receptor levels in tissues. However, in our immunostaining analysis, we did not find any association between adipocytokine expression and BMI. This finding was consistent with the idea that peritumoral adipose tissues are a key contributor to circulating leptin levels and tumor leptin production and act as a minor source of blood adipocytokine [23]. Because BMI is the best proxy for body fat percentage among ratios of weight and height and is generally used as a vague means of estimating adiposity, BMI could be related to adipocytokine levels in blood but not in tissues. This assumption is supported by the observation of Hancke et al. [30] who found a strong positive association between leptin levels and BMI.
Our present study has several limitations. First, we could not evaluate adipocytokine expression in peritumoral normal breast tissue, because TMA was used. So, our results were limited to tumor tissues and a comparative study of the adipocytokine expression in breast cancer with a control group, including normal or benign breast tissue between cancer tissue, may be necessary to understand adipocytokine effects. Second, we could not evaluate adipocytokine expression in breast cancer tissue and blood samples simultaneously. Although we assumed that the level of serum adipocytokine is not correlated with tissue adipocytokine expression based on multiple studies [17,19,23,28], analysis of serum adipocytokine may be necessary to form a solid conclusion. Third, the adipocytokine action mechanism in breast tissue was not studied, which can be further clarified through in vitro experiments. Despite these limitations, we believe that this study is meaningful because, it provided evidence that leptin is inversely related to cancer invasion, and that adiponectin is significantly related to breast cancer invasion.
Figures and Tables
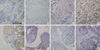 | Figure 1Representative examples of immunohistochemical expression of leptin (A, E), leptin receptor (B, F), adiponectin (C, G), and adiponectin receptor (D, H) in invasive ductal carcinoma (upper row) and ductal carcinoma in situ (lower row). Staining for leptin, leptin receptor, adiponectin, and adiponectin receptor appears in the cytoplasm of tumor cells. We interpreted the results semi-quantitatively based on the number of stained cells and signal intensity (immunohistochemical staining, ×200). Scale bar means 200 micrometer. |
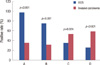 | Figure 2Comparison of leptin (A), leptin receptor (obR) (B), adiponectin (C), and adiponectin receptor (AdipoR) (D) expression levels between patients with ductal carcinoma in situ (DCIS) and invasive breast cancer. Expression of leptin and ObR were significantly higher in the DCIS group than in the invasive cancer group, and adiponectin and AdipoR expression was significantly higher in the invasive cancer group than the DCIS group. |
Table 2
Clinicopathologic characteristics related to adipocytokines and their receptor expression in breast cancer tissues

References
1. Reinier KS, Vacek PM, Geller BM. Risk factors for breast carcinoma in situ versus invasive breast cancer in a prospective study of pre- and post-menopausal women. Breast Cancer Res Treat. 2007. 103:343–348.

3. Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003. 95:1218–1226.

4. Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004. 363:1346–1353.

5. Vona-Davis L, Rose DP. Angiogenesis, adipokines and breast cancer. Cytokine Growth Factor Rev. 2009. 20:193–201.

6. Housa D, Housová J, Vernerová Z, Haluzík M. Adipocytokines and cancer. Physiol Res. 2006. 55:233–244.

8. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994. 372:425–432.

9. Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002. 94:1704–1711.

10. Jardé T, Caldefie-Chézet F, Damez M, Mishellany F, Perrone D, Penault-Llorca F, et al. Adiponectin and leptin expression in primary ductal breast cancer and in adjacent healthy epithelial and myoepithelial tissue. Histopathology. 2008. 53:484–487.

11. Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004. 10:4325–4331.

12. Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, Argiles JM, et al. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000. 5:421–426.

14. Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O'Regan RM, et al. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008. 68:9712–9722.

15. Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006. 12:1447–1453.

16. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995. 83:1263–1271.

17. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999. 257:79–83.

18. Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003. 9:5699–5704.
19. Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004. 89:1102–1107.

20. Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007. 13:332–339.

21. Taliaferro-Smith L, Nagalingam A, Zhong D, Zhou W, Saxena NK, Sharma D. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene. 2009. 28:2621–2633.

22. McMurtry V, Simeone AM, Nieves-Alicea R, Tari AM. Leptin utilizes Jun N-terminal kinases to stimulate the invasion of MCF-7 breast cancer cells. Clin Exp Metastasis. 2009. 26:197–204.

23. Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007. 14:189–206.

24. Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2). J Biol Chem. 2006. 281:26320–26328.

25. Chen C, Chang YC, Liu CL, Chang KJ, Guo IC. Leptin-induced growth of human ZR-75-1 breast cancer cells is associated with up-regulation of cyclin D1 and c-Myc and down-regulation of tumor suppressor p53 and p21WAF1/CIP1. Breast Cancer Res Treat. 2006. 98:121–132.

26. Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, et al. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep. 2008. 20:971–977.

27. Arditi JD, Venihaki M, Karalis KP, Chrousos GP. Antiproliferative effect of adiponectin on MCF7 breast cancer cells: a potential hormonal link between obesity and cancer. Horm Metab Res. 2007. 39:9–13.

28. Karaduman M, Bilici A, Ozet A, Sengul A, Musabak U, Alomeroglu M. Tissue levels of adiponectin in breast cancer patients. Med Oncol. 2007. 24:361–366.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download