Abstract
Purpose
Hypoxia, which is a loss of oxygen in tissues, is a common condition in solid tumors due to the tumor outgrowing existing vasculature. Under hypoxic conditions, hypoxia-inducible factor (HIF)-1α rapidly accumulates and transactivates hundreds of genes, such as matrix metalloproteinases (MMPs). MMPs contribute to invasion and metastasis of tumor cells by degrading the surrounding basement membrane and extracellular matrix barriers, which enables the easy migration and spread of cancer cells. We examined whether hypoxia increases tumor cell invasion, and whether increased invasiveness was due to HIF-1α and MMP-9 expression.
Methods
Transwell invasion assays were performed to demonstrate whether hypoxia enhance tumor invasion by use of MDA-MB-231 breast cancer cells. An immunofluorescence assay was used to demonstrate expression of HIF-1α and MMP-9 under hypoxic conditions. Luciferase and ChiP assays were performed to demonstrate that MMP-9 promoter activity was regulated by HIF-1α.
Results
HIF-1α was stabilized under hypoxic conditions and stimulated MMP-9 expression, which affected the tumor invasiveness of breast cancer cells. HIF-1α transactivated the MMP-9 promoter by forming a transcriptional unit with p300, thus increasing expression of MMP-9 transcripts. Zymography indicated that MMP-9 had more gelatinase activity under hypoxic conditions than normoxic conditions. Furthermore, the small GTPase Ras was also activated in response to hypoxia, which then aids stabilization of HIF-1α, and in turn upregulates MMP-9 expression. We also demonstrate that MMP-9 is upregulated concurrently with HIF-1α in tumor tissues from patients with breast cancer.
Hypoxia, which is a loss of oxygen in tissues, is a common condition in solid tumors that occurs when tumors outgrow existing vasculature [1,2]. Oxygen tension is approximately 7% in normal tissues and 1.3% in solid tumors [3,4]. Tumor cells can survive under hypoxic conditions by adapting to low oxygen tension or by increasing vascularization. Among the genes involved in tumor angiogenesis, vascular endothelial growth factor (VEGF) is the most investigated and anti-VEGF antibody (Avastin™) is now available to treat various cancers.
Another major regulator used by cancer cells to adapt to hypoxia is the transcription factor hypoxia-inducible factor-1 (HIF-1). HIF-1 is composed of an HIF-1α and an HIF-1β subunit. Unlike the oxygen regulated HIF-1α subunits, HIF-1β is constitutively expressed and not responsive to oxygen concentrations. Of the HIF-1 subunits, HIF-1α is the most ubiquitously expressed, and functions as the master oxygen homeostasis regulator of in many cell types [5,6]. Under normoxic conditions, HIF-1α is hydroxylated and binds to von Hippel-Lindau tumor suppressor protein (pVHL), which in turn targets HIF-1α for degradation [7,8]. In contrast, HIF-1α rapidly accumulates and transactivates hundreds of genes under hypoxic conditions, including angiogenic and growth factors and receptors, and extracellular proteases such as matrix metalloproteinases (MMPs) [9,10].
MMPs are endopeptidases that degrade most of the components of the extracellular matrix (ECM), including the basement membrane (BM). MMPs are overexpressed in various solid tumors, including breast cancer, and are recognized to play important roles in many steps of the metastatic process. MMPs contribute to invasion and metastasis of tumor cells by degrading the surrounding BM and ECM barriers, which then enables the easy migration and spread of cancer cells. MMPs are also involved in the regulation of cell proliferation, release of growth factors and angiogenesis [11,12]. MMP activity is controlled by the proteolytic cleavage of inactive proforms into mature active forms and by interaction with the endogenous tissue inhibitors of metalloproteinases.
HIF-1α is overexpressed in primary breast cancers [13]. Increased MMP expression and activation are correlated with increased metastatic potential and poor prognosis in breast cancer [14,15]. Recent studies have also correlated HIF-1α with VEGF gene expression in human breast cancer [16]. Furthermore, HIF-1α is upregulated regardless of human epidermal growth factor receptor 2 (HER2)/c-erb2 expression or estrogen receptor (ER) status [13,17].
Hypoxia stimulates tumor angiogenesis and metastasis via HIF-1α [3,10]. Many reports suggest that hypoxia and HIF-1 upregulate expression and/or activity of MMPs, and that a high level of HIF-1α at diagnosis is predictive of early relapse and metastatic disease, and correlates with poor outcomes [18,19]. In many cancer tissues and cells, MMPs are differentially expressed and have various functions [20,21]. However, little is known about how HIF-1α regulates MMP expression to enhance tumor cell invasion. Among MMPs, MMP-9 has been positively correlated with a higher incidence of metastases in breast or lung cancer [20,22,23]. In this study, we examined whether hypoxia increases tumor cell invasion, whether increased invasiveness is due to MMP-9 expression, and examined the role that the Ras gene plays in the process.
CoCl2, desferoxamine (DFO), and MG132 were purchased from Sigma Chemical Co. (St Louis, USA). Antibodies against HIF-1α, MMP-9, p-ERK, ERK and tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, USA). Antibodies against Ras and GST-Raf1-PBD were purchased from Upstate Biotechnology (Lake Placid, USA). TRITC- and FITC-conjugated secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, USA).
The MDA-MB-231 breast cancer cell line and NCI-H-1299 lung cancer cell line were grown in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum at 37℃ and 5% CO2.
Hypoxic conditions were created in an airtight chamber deoxygenated with the constant infusion of a hypoxic gas mixture (37℃, 5% CO2, 1% O2, 94% nitrogen). The O2 content was monitored with an O2-analyzer. Similarly, normoxic conditions were maintained in a standard incubator (37℃, 5% CO2, 20% O2, balanced N2).
Matrigel invasion assays were performed using modified Boyden chambers with polycarbonate nucleopore membranes (Corning, USA). CoCl2 and DFO were used to create hypoxic conditions. First, pre-coated filters (6.5 mm in diameter, 8-µm pore size, Matrigel 100 µg/cm2) were rehydrated with 100 µL media. Cells in 200 µL serum-free medium were seeded into the upper part of the chamber, and the lower compartments were filled with 1 mL serum-free media. Following a 24-hour incubation at 37℃, non-invaded cells on the upper surface of the filter were wiped off with a cotton swab, and the invaded cells on the lower surface of the filter were fixed and stained with hematoxyline. Invasiveness was determined by counting cells in four microscopic fields per well, and the extent of invasion was expressed as an average number of cells per microscopic field.
Total RNA was extracted using TriZol reagent (Invitrogen Life Technologies, Carlsbad, USA) and used for reverse transcription polymerase chain reaction (RT-PCR). Briefly, RNA was reverse-transcribed with the Murine-Moloney leukemia virus using oligo-dT primer. PCR was performed over 30 cycles of sequential reactions: 94℃ for 30 seconds, 55℃ for 30 seconds, and 72℃ for 30 seconds. Oligonucleotide primers were purchased from Bioneer (Seoul, Korea). PCR primer sequences are described in Table 1.
Cell extracts for Western blotting were prepared in modified RIPA buffer (50 mM Tris-HCl [pH 7.4], 1% Nonidet P-40 [NP-40], 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM Na3VO4, 1 mM NaF, 2 mM phenylmethylsulfonylfluoride, 100 µg/mL leupeptin, 10 µg/mL pepstatin, 1 µg/mL aprotinin, and 2 mM EDTA). Proteins in lysates were separated by SDS-PAGE and transferred to a nitrocellulose membrane. After transfer, the membrane was soaked in a blocking buffer (20 mM Tris base [pH 7.5], 137 mM NaCl, 0.1% Tween 20, and 5% nonfat milk) to prevent nonspecific binding, incubated with primary antibodies for 1 hour at room temperature, and then washed with TBS-T. Then, the membrane was incubated with peroxidase-conjugated secondary antibodies, washed, and visualized using an ECL system (Amersham, Piscataway, USA).
MDA-MB-231 cells were incubated under normoxic or hypoxic conditions for 24 hours and the cell culture medium samples were collected. Samples were loaded on denaturing 8% polyacrylamide-SDS-gels containing 0.1% gelatin to measure gelatinase activity. Gels were washed in distilled water and incubated in 2.5% Triton-X100 for 1 hour to remove SDS, and then incubated overnight at 37℃ in an assay buffer (50 mM Tris-HCl [pH 7.3], 200 mM NaCl, 5 mM CaCl2, and 0.02% Brij-35). The reaction was stopped by staining the gels with 0.1% Coomassie Brilliant Blue and the gelatinase activity was detected as clear bands against the aquablue stained gelatin background.
MDA-MB-231 and NCI-H-1299 cells were plated on slides. After an incubation under hypoxic conditions, cells were pre-incubated with solutions containing 5% normal goat serum, 2% BSA, and 0.1% Triton X-100 for 1 hour at room temperature. MMP-9 (1:500) or HIF-1α (1:500) antibodies were used. Cells were incubated overnight at 4℃ with each antibody, and then washed and incubated for 1 hour with TRITC- and FITC-conjugated secondary antibody (1:200) at room temperature. Images were captured using an imaging system (KAPPA ImageBase DX 30; KAPPA Opto-Electronic Inc., Boca Raton, USA).
MDA-MB-231 cells were incubated for 1 hour in a hypoxia chamber, and lysed with lysis/binding/wash buffer (25 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM MgCl2, 1% NP-40, 1 mM DTT, and 5% glycerol), and then GST-Raf1-PBD conjugated to glutathione-agarose beads was added to the cell lysates to collect activated Ras. After a 1 hour incubation at 4℃, the beads were washed with lysis/binding/wash buffer, resuspended in 2× SDS sample buffer, and electrophoresed on 12% SDS-PAGE. Ras-GTP was detected by Western blotting using a mouse monoclonal anti-Ras-antibody.
MDA-MB-231 cells were transiently transfected with MMP-9-Luc using Lipofectamine reagents, and all clones were cotransfected with pCMV-β-galactosidase to normalize cell number and transcription variation. After 24 hours, the cells were washed with ice-cold PBS and lysed with 100 µL of lysis buffer. To measure luciferase activity, 20 µL of cell extracts were mixed in 100 µL of assay buffer, and read with a luminometer (Berthold Lumat LB9501; Berthold Technologies, Oak Ridge, USA). Luciferase activity was normalized by measuring the β-galactosidase activity at 420 nm.
MDA-MB-231 cells were incubated under hypoxic conditions for 6 hours, fixed with formaldehyde, and sonicated to prepare the chromatin sample. Chromatin samples were immunoprecipitated with antibodies against HIF-1α, p300, Ac-H3, Ac-H4 or normal rabbit IgG antibodies at 4℃ for 3 hours. Immunoprecipitated DNA was amplified by PCR.
Hypoxia-induced MDA-MB-231 cells showed marked invasion that increased by more than 7-fold compared to cells under normoxic conditions (Figure 1). We examined whether hypoxic conditions increased MMP expression. MMP-1, MMP-2, MMP-3, MMP-9, TIMP2, and TIMP3 were all silent under normoxic conditions, but were highly transcribed under hypoxic conditions, suggesting that hypoxia enhances tumor invasion by inducing MMP expression (Figure 2).
MDA-MB-231 and NCI-H-1299 cells were incubated in the presence or absence of CoCl2 or DFO, and total proteins were extracted and analyzed by Western blotting using HIF-1α or MMP-9 antibody. CoCl2 or DFO enhanced HIF-1α and MMP-9 induction in both cell lines (Figure 3A).
Transfected HIF-1α wt DNA construct was incubated in the presence of protease inhibitor, MG132 to demonstrate whether HIF-1α directly induced MMP-9 expression. As shown in Figure 3B, CoCl2 induced HIF-1α and MMP-9 expression, and HIF-1α wt-expressing cells incubated with MG132 showed enhanced HIF-1α and MMP-9 induction. An immunofluorescence assay was used to confirm expression of HIF-1α and MMP-9 under hypoxic conditions. HIF-1α and MMP-9 immunoreactivity were found under hypoxic conditions, but not normoxic conditions (Figure 4). As MMP-9 acts by cleavage from pro-MMP-9, a zymography assay was used to test MMP-9 activity in hypoxic condition. MMP-9 activity was also higher under hypoxic conditions, as previously described (Figure 3C).
As shown in Figure 5A, hypoxia resulted in the activation of Ras and the downstream signaling molecule, p42/p44, in MDA-MB-231 cells. To confirm the relationship between Ras, HIF-1α and MMP-9, transfected HIF-1α wt, SiHIF-1α, Ras DN or Ras Ac were incubated in the presence or absence of MG132 or CoCl2. HIF-1α and MMP-9 were enhanced in Ras Ac-expressing cells without CoCl2, indicating that Ras contributes to HIF-1α stabilization and leads to MMP-9 gene expression. But, MMP-9 was not induced by CoCl2 in SiHIF-1α- or Ras DN-expressing cells (Figure 5B).
Based on the results above, we found that hypoxia induced MMP-9 expression at the transcriptional level via Ras and HIF-1α. To further confirm whether Ras and HIF-1α regulate MMP-9 promoter activity under hypoxic conditions, we measured MMP-9 promoter activity in MDA-MB-231 and NCI-H-1299 cells. MDA-MB-231 cells were transiently transfected with a MMP-9 luciferase reporter gene, and HIF-1α wt, SiHIF-1α, Ras Ac or Ras DN were used to evaluate its role in MMP-9 gene induction. MMP-9 promoter activity was higher under hypoxic conditions, and MMP-9 showed the same promoter activity when HIF-1α was stabilized by incubating with MG132, even under normoxic conditions. When HIF-1α was not expressed using SiHIF-1α, MMP-9 promoter activity was low, indicating that MMP-9 promoter activity was regulated by HIF-1α. Furthermore, MMP-9 promoter activity was higher in Ras Ac-expressing cells than in Ras DN-expressing cells, suggesting that Ras-GTP contributes to MMP-9 promoter activity via HIF-1α stabilization (Figure 6A).
NCI-H-1299 cells were also examined, and similar results were observed to those of MDA-MB-231 cells (data not shown). We performed ChIP assays to further examine whether hypoxia changes the chromatin conformation of the MMP-9 gene promoter. MDA-MB-231 cells were incubated under hypoxic conditions for 6 hours, and chromatin was immunoprecipitated with rabbit IgG, HIF-1α, p300, acetylated-H3 histone or acetylated-H4 histone antibody. We found that hypoxia recruited HIF-1α to the MMP-9 promoter with p300, and led to histone modification via acetylation of histone H3 and H4 (Figure 6B).
To reaffirm that HIF-1α contributes to MMP-9 gene induction, we tested the HIF-1α and MMP-9 gene expression level in tumor tissue from patients with breast cancer. Among four patients, three samples showed that HIF-1α and MMP-9 were induced in tumor tissue, but not in normal tissue. These results suggest that HIF-1α is necessary for MMP-9 gene expression in tumors (Figure 7).
In the past, hypoxia was only considered to be a predictor of poor response to radiotherapy or chemotherapy. However, many clinical and experimental studies have shown an association between the hypoxic tumor microenvironment, nutrient deprivation, and the metastatic process of various solid tumors. HIF-1 complex, which is composed of a heterodimer pair of HIF-1α and HIF-1β, is a newly researched molecule. When cells are hypoxic,-(almost all solid tumors are hypoxic)-, HIF-1α hydroxylation and pVHL association is lowered and HIF-1α protein levels rise. HIF-1α protein, with its nuclear localization sequence, is then translocated to the nucleus, where it heterodimerizes with HIF-1β. The HIF-1α complex binds to hypoxic responsive elements and upregulates hypoxic-regulated genes, inducing changes that enable tumor cells to survive apoptosis, angiogenesis, invasion and metastases [3].
In this study, hypoxia-induced MDA-MB-231 cells showed increased invasiveness compared to cells under normoxic conditions. Several studies have described the transcriptional upregulation of proteases such as MMP-9 as a mechanism responsible for hypoxia-induced cell invasion and metastasis [24,25] MMP-9 overexpression is positively correlated with a higher incidence of metastasis in breast and lung cancers [20,22,23]. The present study showed that MMP-9 expression was upregulated by HIF-1α in the MDA-MB-231 breast cancer cell line, NCI-H-1299 lung cancer cell line, and breast cancer tissue. This means that MMP-9 is an essential component of hypoxia-induced invasion in breast cancer cells. We have also shown that, hypoxic microenvironments in human breast tumors are correlated with MMP-9 activity. Large breast tumors may have more severe hypoxic microenvironments that induce cellular invasive properties via MMP-9 activation mechanisms, suggesting a higher incidence of metastasis in large primary tumors. Quantified data on the expression of MMP-9 in various sizes of tumors are needed to evaluate this hypothesis.
In contrast, the Ras gene is involved in this process. The Ras oncogene is active and relevant in about 30% of human cancers, and it plays a role in tumor cell invasion [26]. Several investigators have recently shown that Ras affects VEGF expression through HIF-1α [27]. This interaction is mediated through tyrosine kinase signaling via murine leukemia viral oncogene homolog 1/map/ERK kinase-1/extracellular signal-regulated kinase (Raf/MEK1/ERK), a pathway shared with AKT [28]. HIF-1α and MMP-9 were enhanced in Ras Ac-expressing cells without CoCl2, indicating that Ras contributes to HIF-1α stabilization and leads to MMP-9 gene expression. To further evaluate whether Ras and HIF-1α regulate MMP-9 promoter activity under hypoxic conditions, we measured the MMP-9 promoter activity using the luciferase and ChIP assays. Our results showed that MMP-9 promoter activity was higher in Ras Ac-expressing cells than in Ras DN-expressing cells, suggesting that Ras-GTP contributes to MMP-9 promoter activity via HIF-1α stabilization.
Based on these results, HIF-1α, MMP-9 and Ras seem to be promising therapeutic targets in breast cancer research. Many approaches have been considered to identify agents that reduce HIF-1α protein levels given the overexpression of HIF-1α in so many tumors and association of HIF-1α with tumor angiogenesis [5,10].
HIF-1α has emerged as an important transcription factor in various tumors, especially in breast cancer tumor biology. In breast cancer, HIF-1α is a logical target for chemoprevention strategies in patients at higher genetic risk for breast cancer. Additionally, even though interactions between estrogen intracellular signaling pathways and HIF-1α are still not fully defined in breast cancer, ER-β downstream factors are candidates for estrogen modulation of HIF-1α levels. In vitro data on breast cancer cell lines shows that c-erbB-2 enhances HIF-1α expression [29]. In a clinical study, c-erbB-2 positivity was related to HIF-1α protein expression and high angiogenesis [30].
Hypoxia promotes tumor invasion, and HIF-1α plays a pivotal role. Under hypoxic conditions, HIF-1α is stabilized and stimulates expression of MMP-9 gene affecting tumor invasion, while Ras is activated, which then aids in stabilizing HIF-1α, which in turn upregulates MMP-9 expression. Additional studies are required to define the interactions between endocrine function, endocrine receptors, and HIF-1 transcriptional factors. Transgenic mouse model experiments and human breast cancer studies may help determine whether HIF-1α inhibitors, including 2ME2, the inhibitor for the Ras pathway, and an MMP-9 inhibitor decrease invasion or metastases of tumors. Furthermore, their chemopreventive effects in genetic high-risk patients should be evaluated.
In conclusion, hypoxia promoted tumor invasion via HIF-1α. HIF-1α was stabilized under hypoxic conditions and stimulated MMP-9 gene expression, thus affecting tumor invasion in several cell lines. HIF-1α trans-activated the MMP-9 promoter by forming a transcriptional unit with p300, and thus, increased further expression of MMP-9 transcripts. Zymography also indicated that MMP-9 has more gelatinase activity under hypoxic conditions than normoxic conditions.
Furthermore, small GTPase Ras was also activated in response to hypoxia, which aided in the stabilization of HIF-1α, and upregulated MMP-9 expression. We demonstrated that MMP-9 was upregulated concurrently with HIF-1α in tumor tissues from patients with breast cancer.
These results suggest that HIF-1α directly promotes cell invasion through a MMP-9 and Ras dependent mechanism and that future anti-tumor agents may target HIF-1α and MMP-9.
Figures and Tables
 | Figure 1Hypoxia enhances tumor cell invasion. (A) MDA-MB-231 cells were incubated in transwell chamber under normoxic and hypoxic conditions for 24 hours, and the cells were stained with hematoxyline. (B) Invaded cells were counted in four microscopic fields per well. |
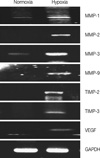 | Figure 2Hypoxia enhances tumor cell invasion through matrix metalloproteinases (MMPs). MDA-MB-231 cells were incubated under normoxic and hypoxic conditions for 24 hours, and then total RNA was isolated. MMP-1, MMP-2, MMP-3, MMP-9, TIMP-2, and TIMP-3 levels were analyzed using reverse transcription polymerase chain reaction. GAPDH was used as a control. |
 | Figure 3Hypoxia induces matrix metalloproteinase-9 (MMP-9) expression via hypoxia-inducible factor (HIF)-1α induction. (A) MDA-MB-231 cells and NCI-H-1299 cells were incubated in the presence or absence of CoCl2 or desferoxamine (DFO), and total proteins were extracted and analyzed by Western blotting using HIF-1α antibody. MMP-9 was detected in culture medium. The data shown are representative of three independent experiments. (B) MDA-MB-231 cells were transfected with HIF-1α wt DNA construct and incubated in the presence of protease inhibitor, MG132. HIF-1α was detected in cell lysates and MMP-9 was analyzed in culture medium by Western blotting. (C) Zymography assay was used to examine MMP-9 activity. MDA-MB-231 cells were incubated under normoxic or hypoxic conditions for 24 hours and cell culture medium samples were collected. Samples were loaded on denaturing 8% polyacrylamide-SDS-gel containing 0.1% gelatin. Gels were washed in distilled water and were incubated in assay buffer, and then were stained with 0.1% Coomassi Brilliant Blue. Clear band marked in 82 kDa shows the gelatinase activity. |
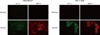 | Figure 4Hypoxia induces matrix metalloproteinase-9 (MMP-9) expression via hypoxia-inducible factor (HIF)-1α induction. Immunofluorescence assay was performed to examine expression of HIF-1α and MMP-9 in cellular level by imaging. Each slide was stained with antibodies against HIF-1α or MMP-9, and then incubated with FITC-conjugated rabbit antibody for HIF-1α or with TRITC-conjugated mouse antibody for MMP-9. |
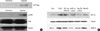 | Figure 5Ras-GTP induces hypoxia-inducible factor (HIF)-1α stabilization, and consequently leads to matrix metalloproteinase-9 (MMP-9) gene expression. (A) MDA-MB-231 cells were incubated in normoxic or hypoxic conditions for 1 hour (upper panel). Ras activity was measured GTP-Ras bound to GST-Ras-PBD fusion protein (low panel). Cell lysates were prepared and Western blotting was performed using antibodies against phospho-ERK or ERK. Tubulin was used as a loading control. (B) MDA-MB-231 cells were transfected with HIF-1α wt, SiHIF-1α, Ras DN or Ras Ac DNA construct and incubated in the presence or absence of CoCl2. Total protein was prepared, and Western blotting was performed with HIF-1α antibody. MMP-9 was detected in culture medium. Tubulin indicated amount of loading. |
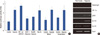 | Figure 6Hypoxia-inducible factor (HIF)-1α and Ras regulates matrix metalloproteinase-9 (MMP-9) promoter activity. (A) MMP-9 luciferase construct was cotransfected with HIF-1α wt, SiHIF-1α, Ras Ac or Ras DN in MDA-MB-231 cells. After 24 hours, cells were incubated in hypoxic condition or treated with MG132. Bars represent the average luciferase activity of four independent experiments. β-galactosidase assay were used to normalize for transfection efficiencies. NCI-H-1299 cells showed the same results as MDA-MB-231 cells (data not shown). (B) MDA-MB-231 cells were incubated in the normoxic or hypoxic conditions for 6 hours, and chromatin was immunoprecipitated with Rabbit IgG, HIF-1α, p300, acetylated-H3 histone or acetylated-H4 histone antibody. Purified DNA was amplified by semiquantitative polymerase chain reaction (PCR) using MMP-9 primers. Five-fold dilutions of DNA were amplified as an internal control of the PCR. |
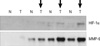 | Figure 7Hypoxia-inducible factor (HIF)-1α plays a role in tumor invasion by inducing matrix metalloproteinase-9 (MMP-9) gene expression in cancer patients. Tissues from patients with breast cancer and corresponding normal tissues were prepared, and lysed. HIF-1α and MMP-9 expression were detected by Western blotting (arrows).
N=normal; T=tumor tissue.
|
References
1. Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002. 2:38–47.
2. Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M. Tumor hypoxia: a target for selective cancer therapy. Cancer Sci. 2003. 94:1021–1028.

3. Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004. 9:Suppl 5. 10–17.

4. Vaupel P, Kelleher DK, Höckel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol. 2001. 28:2 Suppl 8. 29–35.

6. Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda). 2004. 19:176–182.

7. Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001. 292:464–468.

8. Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001. 292:468–472.

9. Semenza GL. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest. 2000. 106:809–812.

10. Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002. 8:4 Suppl. S62–S67.

11. Freije JM, Balbín M, Pendás AM, Sánchez LM, Puente XS, López-Otín C. Matrix metalloproteinases and tumor progression. Adv Exp Med Biol. 2003. 532:91–107.

12. Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000. 18:1135–1149.

13. Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999. 59:5830–5835.
14. Duffy MJ, Maguire TM, Hill A, McDermott E, O'Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000. 2:252–257.

15. Jones JL, Glynn P, Walker RA. Expression of MMP-2 and MMP-9, their inhibitors, and the activator MT1-MMP in primary breast carcinomas. J Pathol. 1999. 189:161–168.

16. Jensen RL, Ragel BT, Whang K, Gillespie D. Inhibition of hypoxia inducible factor-1alpha (HIF-1alpha) decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. J Neurooncol. 2006. 78:233–247.

17. Bos R, van Diest PJ, van der Groep P, Shvarts A, Greijer AE, van der Wall E. Expression of hypoxia-inducible factor-1alpha and cell cycle proteins in invasive breast cancer are estrogen receptor related. Breast Cancer Res. 2004. 6:R450–R459.
18. Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, et al. Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003. 97:1573–1581.

19. Gruber G, Greiner RH, Hlushchuk R, Aebersold DM, Altermatt HJ, Berclaz G, et al. Hypoxia-inducible factor 1 alpha in high-risk breast cancer: an independent prognostic parameter? Breast Cancer Res. 2004. 6:R191–R198.

20. Pritchard SC, Nicolson MC, Lloret C, McKay JA, Ross VG, Kerr KM, et al. Expression of matrix metalloproteinases 1, 2, 9 and their tissue inhibitors in stage II non-small cell lung cancer: implications for MMP inhibition therapy. Oncol Rep. 2001. 8:421–424.

21. Zhang X, Zhu S, Luo G, Zheng L, Wei J, Zhu J, et al. Expression of MMP-10 in lung cancer. Anticancer Res. 2007. 27(4C):2791–2795.
22. Hanemaaijer R, Verheijen JH, Maguire TM, Visser H, Toet K, McDermott E, et al. Increased gelatinase-A and gelatinase-B activities in malignant vs. benign breast tumors. Int J Cancer. 2000. 86:204–207.

23. Incorvaia L, Badalamenti G, Rini G, Arcara C, Fricano S, Sferrazza C, et al. MMP-2, MMP-9 and activin A blood levels in patients with breast cancer or prostate cancer metastatic to the bone. Anticancer Res. 2007. 27(3B):1519–1525.
24. Canning MT, Postovit LM, Clarke SH, Graham CH. Oxygen-mediated regulation of gelatinase and tissue inhibitor of metalloproteinases-1 expression by invasive cells. Exp Cell Res. 2001. 267:88–94.

25. Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003. 63:1138–1143.
26. Hernández-Alcoceba R, del Peso L, Lacal JC. The Ras family of GTPases in cancer cell invasion. Cell Mol Life Sci. 2000. 57:65–76.

27. Nguyen M, McCombs MM, Ghandehari S, Kim A, Wang H, Barsky SH, et al. An update on core needle biopsy for radiologically detected breast lesions. Cancer. 1996. 78:2340–2345.

28. Klimberg VS. Advances in the diagnosis and excision of breast cancer. Am Surg. 2003. 69:11–14.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download