Abstract
Purpose
Chemotherapies for breast cancer generally have strong cellular cytotoxicity and severe side effects. Thus, significant emphasis has been placed on combinations of naturally occurring chemopreventive agents. Silibinin is a major bioactive flavonolignan extracted from milk thistle with chemopreventive activity in various organs including the skin, prostate, and breast. However, the mechanism underlying the inhibitory action of silibinin in breast cancer has not been completely elucidated. Therefore, we investigated the effect of silibinin in MCF-7 human breast cancer cells and determined whether silibinin enhances ultraviolet (UV) B-induced apoptosis.
Methods
The effects of silibinin on MCF-7 cell viability were determined using the MTT assay. The effect of silibinin on PARP cleavage, as the hallmark of apoptotic cell death, and p53 protein expression in MCF-7 cells was analyzed using Western blot. The effect of silibinin on UVB-induced apoptosis in MCF-7 cells was analyzed by flow cytometry.
Results
A dose- and time-dependent reduction in viability was observed in MCF-7 cells treated with silibinin. Silibinin strongly induced apoptotic cell death in MCF-7 cells, and induction of apoptosis was associated with increased p53 expression. Moreover, silibinin enhanced UVB-induced apoptosis in MCF-7 cells.
Conclusion
Silibinin induced a loss of cell viability and apoptotic cell death in MCF-7 cells. Furthermore, the combination of silibinin and UVB resulted in an additive effect on apoptosis in MCF-7 cells. These results suggest that silibinin might be an important supplemental agent for treating patients with breast cancer.
Breast cancer is the most common cause of cancer death in women [1]. Systemic treatments for breast cancer include cytotoxic, hormonal, and immunotherapeutic agents. Although many anti-cancer therapies are clinically applicable, they generally have strong cellular cytotoxicity and severe side effects [2]. To develop novel strategies that increase the therapeutic efficacy and minimize the systemic toxicity of chemotherapeutic agents, more efforts are being directed towards investigating dietary supplements and other phytotherapeutic agents for their synergistic efficacy with anticancer drugs. Silibinin is one of these agents that has shown chemopreventive and anticancer effects in various studies [3-6].
Silibinin is the major active constituent of silymarin, which is a mixture of flavonolignans extracted from blessed milk thistle (Silybum marianum). Milk thistle extracts have been used for centuries in traditional medicine, and they are widely consumed herbal remedies with several putative beneficial effects on health, such as hepatoprotective properties. Consuming silibinin is safe and non-toxic in animals and humans, as no substantial adverse effects have been reported when silibinin is given to humans and rodents at doses as high as 1% (w/w) or 2 g/kg body weight [7,8]. Many studies have shown that silibinin blocks experimentally induced malignancies of the prostate, skin, and colon [8-10]. Human trials of silibinin are underway for treating prostate cancer, and a completed phase I study has shown no toxic effects [11]. Silibinin plays an important role in breast cancer cells [2,12]. However, the molecular mechanisms associated with the chemopreventive effects of silibinin have not been clearly and systemically elucidated for breast cancer. Therefore, we initiated studies to determine whether silibinin induces apoptosis in breast cancer cells.
Ultraviolet (UV) radiation comprises UVA (320-400 nm) and UVB (280-320 nm), the latter being crucial for inducing cutaneous malignancy and suppressing the immune system. UVB also activates various signal transduction pathways and induces the expression of several specific genes [13]. Lower doses of UVB cause DNA mutations leading to tumor initiation, whereas high doses result in irreparable DNA damage causing apoptosis and eventually cell death [14]. Ecological studies have suggested a possible association between to UVB radiation and reduction in the risk of breast cancer [15]. In a recent study, UV irradiation induced apoptosis in MCF-7 cells [16]. Furthermore, Silibinin enhances apoptosis in response to UVB-induced moderate/excessive damage in spontaneously immortalized human keratinocyte HaCaT cells [17], indicating that silibinin may enhance UVB-induced apoptosis in breast cancer cells. Thus, we investigated whether the combination of silibinin and UVB would affect apoptosis in MCF-7 human breast cancer cells. In this study, we established an experimental breast cancer model to examine the effect of silibinin in MCF-7 cells and explored the possibility of using silibinin as a supplemental agent for patients with breast cancer.
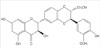
Antibodies against PTEN and p53 were purchased from Santa Cruz Biotechnology (Santa Cruz, USA). Anti-PARP antibody was purchased from Cell Signaling Technology (Beverly, USA). Fetal bovine serum (FBS) and charcoal-dextran treated FBS were obtained from Gibco BRL (Life Technologies, Grand Island, USA). Silibinin (2,3-dihydro-3-[4-hydroxy-3-methoxyphenyl]-2-[hydroxymethyl]-6-[3,5,7-trihydroxy-4-oxobenzopyran-2-yl]benzodioxin), Hanks balanced salt solution, Dulbecco's Modified Eagle's Medium (DMEM), phenol red-free DMEM, and β-actin antibody were obtained from Sigma Chemical Co.(St. Louis, USA).
A human breast cancer cell line (MCF-7) was obtained from the American Type Culture Collection (Rockville, USA). The cells were cultured in DMEM containing 10% FBS, 2 mM glutamine, antibiotics (penicillin G, 60 mg/L; streptomycin, 100 mg/L; amphotericin B, 50 µL/L) under a humid atmosphere (37℃, 5% CO2, 95% air).
The effect of silibinin on MCF-7 cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma) assay. Briefly, 2×l04 cells/well were treated with various concentrations of silibinin (0-200 µM). After a 24-hour incubation the cells were washed twice with phosphate buffered saline (PBS) and MTT (0.5 mg/mL PBS) was added to each well and incubated at 37℃ for 30 minutes. The formazan crystals that formed were dissolved by adding dimethyl sulfoxide (100 µL/well), and the absorbance was read at 570 nm using a microplate reader (Model 3550; BIO-RAD, Richmond, USA). The reduction in cell viability after silibinin treatment was expressed in terms of control (nonsilibinin treated) cells.
After washing the cell pellets with PBS and harvesting, they were lysed with lysis buffer. After a 30-minute incubation at 4℃, cellular debris was removed by centrifugation at 10,000×g for 30 minutes, and the supernatants were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Electrophoretic transfer from the slab gel to nitrocellulose paper and subsequent immunoblotting was performed by incubating the gels with primary antibodies against PARP, PTEN, p53, and β-actin followed by further incubation with HRP-conjugated secondary antibody. Reactive proteins were detected using the enhanced chemiluminescence system (ECL; Amersham Life Sciences, Arlington Heights, USA).
We used a UVB cross-linker (6×8 W, 312 nm, Model CL-508M; Vilber Lourmat, Paris, France) for UVB irradiation. Briefly, serum-starved confluent cells were rinsed twice with PBS, and all irradiations were performed under a thin layer of PBS. Immediately after irradiation, fresh serum-free medium was added to the cells. Mock-irradiated controls followed the same schedule of medium changes without UVB irradiation.
To analyze the apoptotic cells, MCF-7 cells were plated in 60-mm dishes and 24 hours later either, exposed to UVB, treated with silibinin alone, or treated with silibinin (200 µM) before UVB (25 mJ/cm2) exposure. At the end of the 24-hour incubation, attached MCF-7 cells were harvested by trypsinization and pooled with floating cells. The cells were then washed twice with ice-cold PBS and stained with FITC-conjugated annexin V and propidium iodide according to the manufacturer's instructions (Invitrogen, Carlsbad, USA). Stained cells were measured at a fluorescence emission wavelengths of 530 nm and 575 nm using a 488 nm excitation wavelength. Apoptotic cells were analyzed by fluorescence activated cell sorting (FACScalibur; BD Biosciences, San Jose, USA).
MTT assays were performed to determine MCF-7 cell viability after the silibinin incubation (for silibinin structure, Figure 1). As shown in Figure 2, treating MCF-7 cells with 200 µM silibinin for 48 hours resulted in no significant effect on cell viability. However, cell viability decreased significantly when the cells were incubated with silibinin for 72 hours. Moreover, exposure of MCF-7 cells to silibinin resulted in a dose dependent loss of cell viability. Cell viability decreased significantly when the cells were incubated with 200 µM silibinin for 72 hours.
PARP cleavage in silibinin-treated and untreated cells were measured to investigate the apoptotic mechanism of silibinin on MCF-7 cells. MCF-7 cells were cultured with various concentrations of silibinin for 72 hours, and PARP cleavage of MCF-7 cells was analyzed by Western blot using a specific anti-PARP antibody. The data showed that 200 µM silibinin strongly induced PARP cleavage (Figure 3).
We measured p53 expression to determine the possible role of p53 in the induction of apoptosis by silibinin in MCF-7 cells. The effect of silibinin on p53 protein expression was determined using Western blot analysis. MCF-7 cells were treated with various concentrations of silibinin (0-200 µM) for 72 hours, and whole cell extracts were used for the p53 Western blot analysis. As shown in Figure 4, silibinin increased the p53 level in a dose-dependent manner. Interestingly, silibinin had no effect on PTEN, known as a tumor suppressor protein, expression in MCF-7 cells.
We investigated whether the combination of silibinin and UVB affected apoptosis in MCF-7 cells. MCF-7 cells were treated with 200 µM silibinin, UVB alone, or in combination for 72 hours to determine the effect of combined silibinin and UVB on apoptosis. We pretreated cells with 200 µM silibinin prior to UVB irradiation and MCF-7 cell viability was measured by the MTT assay. As shown in Figure 5, UVB induced a significant loss of MCF-7 cell viability. Cell viability in silibinin- pretreated cells was significantly less than with silibinin alone. Florescence staining was performed to determine whether a decrease in MCF-7 cell viability after silibinin treatment and UVB irradiation was the result of apoptosis. Apoptosis in MCF-7 cells was further quantitatively analyzed by flow cytometry. As shown in Figure 6, UVB resulted in only 4.8% apoptotic cells compared with the control; however, silibinin pre-treatment resulted in 8.8% apoptotic cells, accounting for a significant enhancement in UVB-induced apoptosis.
Chemotherapeutic agents have been developed to counter the continuing breast cancer problem. However, most chemotherapeutic drugs effectively target rapidly dividing cells causing damage and are thus referred to as "cytotoxic drugs." These cytotoxic drugs cause a wide variety of side-effects, such as nausea, immunosuppression, myelosuppression, cardiotoxicity, hepatotoxicity, nephrotoxicity, and non-specific neurocognitive problems. Cytotoxic drugs could face limited clinical application due to their high toxicity and increased chemoresistance, so combination therapy/prevention is gaining increased attention as an effective alternative to increase therapeutic efficacy and minimize the systemic toxicity of these chemotherapeutic agents.
Interest in naturally occurring products is increasing for the prevention of carcinogenesis. Based on this idea, certain foods such as vegetables, fruits, and grains, as well as other phytotherapeutic agents offer high anticancer efficacy and low toxicity to normal tissue. One such dietary agent is silibinin, which has a wide range of pharmacological effects, such as inhibition of DNA synthesis, cell proliferation, cell cycle progression, and apoptosis in various cancer cell lines, including breast cancer [3-6]. Moreover, the administration of this compound to various animals has been shown to be nontoxic in many studies [18,19]. However, the molecular mechanisms associated with the anticancer effects of silibinin have not been clearly elucidated for breast cancer. The inhibition of cell viability by silibinin requires p53 protein expression, and p53-deficient cells are less susceptible to silibinin-induced apoptosis [20]. Therefore, we established an experimental breast cancer model to examine the effect of silibinin in MCF-7 cells, which express wild-type p53.
In this study, MTT assays were performed to determine cell viability. No effect of low concentrations of silibinin (≤100 µM) on cell viability was observed. However, at high silibinin concentrations (≥200 µM), cell viability decreased significantly to approximately 60% of control levels. These findings suggest that the effect of silibinin on MCF-7 cells was dose dependent. In 1999, Bhatia et al. [21] reported that treating of prostate, breast, and cervical carcinoma cells with silibinin results in a highly significant inhibition of cell growth and DNA synthesis in a time-dependent manner with a large loss of cell viability only in the case of cervical carcinoma cells. Tyagi et al. [3] reported that silibinin alone results in growth inhibition in a dose-and time-dependent manner. Moreover they suggested that silibinin synergizes with conventional cytotoxic agents during breast cancer treatment. Recently, several studies reported the mechanism underlying the cytotoxicity of silibinin in breast cancer [2,12].
Apoptosis is a distinctive form of cell death, resulting in the death of specific cell populations during physiological processes [22,23]. Inhibiting apoptosis is a possible mechanism of tumor development, and many chemopreventive agents act through the induction of apoptosis to inhibit the carcinogenic process. Therefore, the induction of apoptosis in breast cancer cells may be one of the mechanisms of the anticancer effect of silibnin. Here, we found that silibinin induced apoptotic cell death in MCF-7 cells. PARP cleavage in silibinin-treated and untreated cells was measured, as PARP cleavage is the hallmark of apoptotic cell death [24]. Silibinin (200 µM) strongly induced PARP cleavage. This finding was similar to another study showing that silibinin strongly induces apoptosis in renal cell carcinoma cells [4].
It has long been recognized that tumor suppressor protein p53 is induced by DNA damage [20]. The resulting increase in p53 leads either to the induction of cell cycle arrest or apoptosis. Thus, functional p53 provides a protective effect against tumor growth [25]. Additionally, several studies have shown that silibinin induces p53-dependent apoptosis in various cancer cells [26,27]. Therefore, we measured p53 expression to determine the possible role of p53 in the induction of apoptosis by silibinin in MCF-7 cells. Silibinin increased the level of p53 in a dose-dependent manner, which may have occurred due to increased half-life. However, silibinin has no effect on PTEN expression in MCF-7 cells [28], indicating that silibinin induces p53 protein expression specifically in breast cancer cells. Further studies are in progress to define the role of p53-independent pathway in the induction of silibinin-induced apoptosis in MCF-7cells.
In 2003, Ferguson et al. [16] reported that apoptosis can be induced in MCF-7 cells after UV treatment. Then, Mohan et al. [29] reported that UVB showed a dose- and time-dependent apoptotic death in human epidermoid carcinoma cells, and that silibinin pre-treatment resulted in an increase in UVB-induced apoptosis. In this study, we found that UVB caused a significant increase in loss of viability and apoptosis in MCF-7 cells. Cell viability was significantly less in silibinin-pretreated cells than in silibinin or UVB irradiated alone cells, and apoptosis was greater. These results suggest that silibinin enhanced UVB-induced apoptosis in MCF-7 cells. These findings encourage further mechanistic and in vivo studies to develop silibinin as a chemopreventive or chemotherapeutic agent that enhances UVB-induced apoptosis in human breast cancers.
Figures and Tables
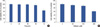 | Figure 2Effects of silibinin on MCF-7 cell viability. (A) Cells were cultured in 96-well plates until 90% confluence and 200 µM silibinin was then added for 24, 36, 48, and 72 hr. The MTT assay was used to detect cell viability. The optical density (O.D.) value of the control was regarded as 100%. Data points are the means±SEs of more than three experiments (p<0.005). (B) Cells were cultured in 96-well plates until 90% confluence and various concentrations of silibinin (0-200 µM) were added for 72 hr. The MTT assay was used to detect cell viability. The O.D. value of the control was regarded as 100%. Data points are the means±SEs of more than three experiments (p<0.005). |
 | Figure 3Effect of silibinin on PARP cleavage in MCF-7 cells. MCF-7 cells (5×106 cells) were treated with various concentrations of silibinin (0-200 µM) for 72 hr, and whole cell extracts were used for Western blot analysis of PARP. β-actin was used as the loading control. |
 | Figure 4Effect of silibinin on p53 and PTEN expression in MCF-7 cells. MCF-7 cells (5×106 cells) were treated with various concentrations of silibinin (0-200 µM) for 72 hr. Cells were lysed with lysis buffer and the amounts of p53/PTEN and β-actin as a loading control were measured by Western blot (A). The relative density of the electrophoretic band was obtained with a LAS-1000 analyzer (Fujifilm, Japan) (B). Data are means±SEs of four separate experiments (p<0.005). |
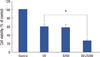 | Figure 5Effect of silibinin and ultraviolet (UV) B on MCF-7 cell viability. MCF-7 cells were treated with 200 µM silibinin and UVB alone or in combination for 72 hr. The MTT assay was used to detect cell viability. The optical density value of the control was regarded as 100%. Data points are the means±SEs of more than three experiments.
*p<0.001 (Student's t-test).
|
ACKNOWLEDGMENTS
The present research was supported in part by the Korea Breast Cancer Foundation, the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (No. 2009-0062917 and M10528010003-05N2801-00310), and the Korea Research Foundation Grant (KRF-2009-0076698).
References
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009. 59:225–249.

2. Lee SO, Jeong YJ, Im HG, Kim CH, Chang YC, Lee IS. Silibinin suppresses PMA-induced MMP-9 expression by blocking the AP-1 activation via MAPK signaling pathways in MCF-7 human breast carcinoma cells. Biochem Biophys Res Commun. 2007. 354:165–171.

3. Tyagi AK, Agarwal C, Chan DC, Agarwal R. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF-7 and MDA-MB 468 cells. Oncol Rep. 2004. 11:493–499.
4. Li L, Gao Y, Zhang L, Zeng J, He D, Sun Y. Silibinin inhibits cell growth and induces apoptosis by caspase activation, down-regulating survivin and blocking EGFR-ERK activation in renal cell carcinoma. Cancer Lett. 2008. 272:61–69.

5. Kaur M, Agarwal R. Silymarin and epithelial cancer chemoprevention: how close we are to bedside? Toxicol Appl Pharmacol. 2007. 224:350–359.

6. Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol Carcinog. 2006. 45:436–442.

7. Wellington K, Jarvis B. Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs. 2001. 15:465–489.

8. Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002. 62:3063–3069.
9. Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997. 89:556–566.

10. Kohno H, Tanaka T, Kawabata K, Hirose Y, Sugie S, Tsuda H, et al. Silymarin, a naturally occurring polyphenolic antioxidant flavonoid, inhibits azoxymethane-induced colon carcinogenesis in male F344 rats. Int J Cancer. 2002. 101:461–468.

11. Flaig T, Agarwal R, Su L, Harrison GS, Gustafson DL, Glode LM. A phase I study of silibinin in hormone refractory prostate cancer. 2005. 23:In : 2005 ASCO Annual Meeting; Abstract #4698.
12. Kim S, Choi JH, Lim HI, Lee SK, Kim WW, Kim JS, et al. Silibinin prevents TPA-induced MMP-9 expression and VEGF secretion by inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine. 2009. 16:573–580.

13. Yarosh DB, Boumakis S, Brown AB, Canning MT, Galvin JW, Both DM, et al. Measurement of UVB-induced DNA damage and its consequences in models of immunosuppression. Methods. 2002. 28:55–62.

14. Kulms D, Schwarz T. Molecular mechanisms of UV-induced apoptosis. Photodermatol Photoimmunol Photomed. 2000. 16:195–201.

15. John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971-1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999. 8:399–406.
16. Ferguson HA, Marietta PM, Van Den Berg CL. UV-induced apoptosis is mediated independent of caspase-9 in MCF-7 cells: a model for cytochrome c resistance. J Biol Chem. 2003. 278:45793–45800.
17. Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Dual efficacy of silibinin in protecting or enhancing ultraviolet B radiation-caused apoptosis in HaCaT human immortalized keratinocytes. Carcinogenesis. 2004. 25:99–106.

18. Vogel G, Trost W, Braatz R, Odenthal KP, Brüsewitz G, Antweiler H, et al. Pharmacodynamics, site and mechanism of action of silymarin, the antihepatoxic principle from Silybum mar. (L) Gaertn. 1. Acute toxicology or tolerance, general and specific (liver-) pharmacology. Arzneimittelforschung. 1975. 25:82–89.
19. Mereish KA, Bunner DL, Ragland DR, Creasia DA. Protection against microcystin-LR-induced hepatotoxicity by Silymarin: biochemistry, histopathology, and lethality. Pharm Res. 1991. 8:273–277.
20. Katiyar SK, Roy AM, Baliga MS. Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol Cancer Ther. 2005. 4:207–216.
21. Bhatia N, Zhao J, Wolf DM, Agarwal R. Inhibition of human carcinoma cell growth and DNA synthesis by silibinin, an active constituent of milk thistle: comparison with silymarin. Cancer Lett. 1999. 147:77–84.

22. Ellis RE, Yuan JY, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991. 7:663–698.

23. Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993. 262:695–700.

25. Finlay CA, Hinds PW, Levine AJ. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989. 57:1083–1093.

26. Dhanalakshmi S, Agarwal C, Singh RP, Agarwal R. Silibinin up-regulates DNA-protein kinase-dependent p53 activation to enhance UVB-induced apoptosis in mouse epithelial JB6 cells. J Biol Chem. 2005. 280:20375–20383.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download