Abstract
Microglandular adenosis (MGA) of the breast is a very rare and benign proliferative lesion. Most patients complain of a palpable breast mass that may arouse a clinical suspicion of breast cancer. Histopathologically, it is hard to distinguish MGA from breast cancer because of the lack of a myoepithelial layer and infiltrative proliferation. Several studies have reported a strong relationship between MGA and carcinoma arising in MGA, so the mass should be excised completely in cases of MGA determined from a core needle biopsy rather than observation. A 72-years-old woman presented with a palpable breast mass. On physical examination, a mass was palpable in the right upper outer quadrant area and somewhat fixed to the surrounding tissues and pectoralis major muscle. We could not detect any mass or dense lesion on mammography because of a grade 4 dense breast. Ultrasonographic findings revealed a low echoic lesion with indistinct margins. The result of a core needle biopsy was MGA, which was confirmed by excision. We report one case of MGA, which was believed to breast cancer clinically.
Microglandular adenosis (MGA) is a very rare disease and has not been reported in Korea. MGA often produces a palpable mass that mimics breast cancer on breast examination. Histologically, small round glands lacking a myoepithelial layer infiltrates normal stroma making it difficult to distinguish MGA from well-differentiated breast cancer. Despite its characteristic infiltrative growth pattern, aggressive local spread or metastasis has not been reported, so it is recognized as a benign lesion. Until now, the treatment choice for MGA is observation, because MGA is known as a benign disease but more recent data show that MGA is highly related to cancer. For that reason, a reassessment of the treatment plan is required for cases in which a core needle biopsy reveals MGA.
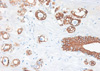
A 72-years-old woman presented with a palpable breast mass. On physical examination, about 2 cm of the mass was palpable at 11 o'clock and 2 cm from the right breast nipple. The mass was somewhat fixed to the surrounding tissues and the pectoralis major muscle. No nipple discharge, dimpling, or skin changes were noted. The mammographic finding was a grade 4 dense breast with benign calcifications in both breasts. A 1.2 cm ill-defined low echoic lesion (BIRADS category 5) was shown in the upper outer quadrant of the right breast on an ultrasonogram (Figure 1). We performed a core needle biopsy to rule out breast cancer. The result of the core needle biopsy was MGA, so we excised the mass completely. The excised mass was about 3×2 cm and 1.2×1 cm of the main mass was shown. The lesion was firm, nodular, and had irregular margins (Figure 2). Microscopic findings revealed many small glands infiltrating the fibrous stroma. The glands were regular, small, and lined by a single epithelial cell layer (Figure 3). Immunohistochemical staining was positive for S-100 protein (Figure 4), and the epithelial cells were negative for estrogen receptor. No evidence of recurrence during a breast examination and ultrasonogram was found at the 6-month follow-up.
MGA of the breast is known as a benign proliferative glandular lesion and was first described in 1968 by McDivitt et al. [1]. MGA can mimic well differentiated breast cancer both clinically and pathologically [2]. All reported patients with MGA are women, ranging in age from 28 to 82 years. Most patients complain of a palpable breast mass or breast thickening that may arouse a clinical suspicion of carcinoma. MGA can also be found incidentally [2]. It is often 3 to 4 cm in size, but occasionally the lesion can be greater than 20 cm, and the margin is usually ill-defined or infiltrative [2]. Mammographic findings of MGA may show increased density or calcifications stimulating breast cancer and lead to a core needle biopsy.
Histologically, MGA is characterized by a haphazard infiltration of small, uniformly open, and round glands in dense, hypocellular, and fibrous tissue, or fatty mammary stroma [3,4]. The glands are lined by a monolayer of flat to cuboidal epithelial cells that lack a myoepithelial layer (Figure 3) [2], which can be verified by immunohistochemistry. MGA immunohistochemistry is positive for pancytokeratin and negative for smooth muscle actin (Figures 5, 6), so the lesion is often confused with a tubular carcinoma. The absence of stromal desmoplasia and the presence of a thickened basement membrane help distinguish MGA from carcinoma [5,6]. MGA has no lobular grouping and frequently overruns normal elements, but it does not invade the perineurium or vasculature. Epithelial cells often lack apical snouts and have vacuolated or clear cytoplasm that contains glycogen [2,7]. The lumens of the glands contain clear or eosinophilic colloid-like secretions, usually positive for PAS and mucicarmine, and occasionally luminal calcification [5,8]. The glands are completely invested by a basement membrane that can be highlighted by type IV collagen, laminin, or PAS staining [7,9]. MGA has no myoepithelial cells, and the epithelial cells are usually positive for cytokeratin and S-100 protein (Figures 4, 5). The epithelial cells are also positive for CAM 5.2, AE-1, and cathepsin D. but negative for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2/neu) [7,8,10,11]. The epithelial cells are also negative for epithelial membrane antigen and gross cystic disease fluid protein 15 as well [5], which is unusual in epithelial breast cancer.
A spectrum of lesions, ranging from typical MGA to atypical MGA and carcinoma arising in MGA (MGACA), has been described [3,11-13]. Atypical MGA (MGA with atypia or AMGA) has features of classic MGA but increased irregularity, density of glands, and cytologic atypia such as hyperchromasia, prominent nucleoli, apoptosis compared to uncomplicated MGA [8]. AMGA shows a more pleomorphic admixture of connected microacini and larger glands and often discloses budding glandular units and luminal bridging. Intraluminal secretions are usually reduced or absent, instead cellular expansion often fills the lumen. Approximately 5-10% of AMGAs have increased Ki67 or p53 labeling, which can be important differential points between MGA and AMGA [8,11,13], as well as important prognostic factors [13]. AMGA probably represents an intermediate lesion in the morphological transition from MGA to invasive carcinoma [8,11,13].
MGACA may show both carcinoma in situ, and invasive carcinoma and has been reported in up to 27% of cases [3,14]. MGA is distinct from mammary carcinoma. The carcinoma component arising in MGA shows various histological features consisting largely of solid nests and glands expanded and filled with cytologically malignant cells. A solid expansile proliferation obliterating the lumen, and severe cytological atypia with frequent apoptotic or mitotic features are observed with an in situ carcinoma [4]. In situ carcinoma tends to retain the underlying alveolar growth pattern of MGA, but invasive foci are formed by the coalescent growth of expanding alveolar of in situ carcinoma elements, associated with a desmoplastic stromal reaction and often accompanied by lymphocytic infiltration [5]. The epithelial cells may exhibit cytoplasmic clearing and glandular changes comparable to those of MGA. Duct forming carcinoma tends to retain the acinar budding pattern of AMGA, but it can be distinguished by its high mitotic rate, more pronounced cytological abnormality, necrosis, and desmoplastic stromal reaction. The basement membrane, which is usually preserved around the glands of MGA and AMGA, tends to be disrupted in MGACA [15].
The immunohistochemical profile of MGACA is similar to MGA itself [5,7]. As previously stated, MGA has no myoepithelial cell layer, and the epithelial cells are usually positive for cytokeratin 7 and S-100 protein (Figure 4) but negative for ER, PR, and HER2/neu. In this context, MGA, AMGA, in situ carcinoma, and invasive carcinoma have a similar immunohistochemical profile [7,8,11,13]. A basement membrane is present in MGA and AMGA, whereas in situ carcinoma is defined by an immunohistochemical study for type IV collagen, laminin, or PAS stain, which is absent in invasive carcinoma [4]. Invasive carcinoma shows a higher percentage of staining for MIB-1 (a cell proliferation marker) and p53 than does AMGA.
Despite its characteristic infiltrative growth pattern, aggressive local spread or metastasis has not been observed, so MGA is classified as a benign proliferative disease. But, although MGA is a benign lesion, if it is not excised completely, it may recur [15]. Whether MGA is a precancerous lesion or innocent bystander can be debated [4]. But, recent studies have found a strong relationship between MGA and MGACA, so the current management for MGA is complete excision, and the excised specimen needs to be sampled thoroughly to rule out the possibility of an associated carcinoma. However, such a relationship is uncertain because of the rarity of the disease. A wide excision with a clear resection margin and careful follow-up is needed for AMGA. Re-excision is recommended in cases with margins positive for AMGA; however, the assessment of surgical margins in carcinomas remains controversial. S-100 staining is helpful to obtain a tumor-free resection margin. The prognosis for MGACA varies [8,13], but most cases of carcinoma arising in MGA have a relatively favorable prognosis despite being ER and PR negative. Adjuvant chemotherapy is recommended for patients with axillary lymph node metastasis [2].
MGA, AMGA, and MGACA display similar pathological and immunohistochemical features, but their prognoses are much different. To mistake MGA for sclerosing adenosis or a tubular adenoma results in undertreatment, whereas misdiagnosing MGA as MGACA results in overtreatment. Therefore, distinguishing MGA from AMGA or MGACA and appropriate management is very important.
In conclusion, MGA of the breast is a very rare benign disease that can mimic breast cancer clinically and pathologically. An MGA mass should be completely excised based on a core needle biopsy result because of its strong relationship with breast cancer.
Figures and Tables
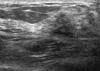 | Figure 1Ultrasonographic finding of microglandular adenosis. Ill-defined low echoic lesion (BIRADS category 5) in the upper outer quadrant of right breast was shown in ultrasonogram. |
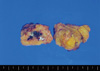 | Figure 2Gross appearance of microglandular adenosis (MGA). This is a gross appearance of MGA. Hematoma in the mass is result of the previous needle biopsy. |
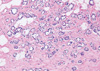 | Figure 3Microscopic finding of microglandular adenosis (MGA). Infiltrating round glands lacking a myoepithelial layer are seen with luminal eosinophilic secrestions. The glands are regular and small, and are lined by single epithelial layer (H&E stain, ×40). |
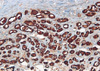 | Figure 4Immunohistochemistry (IHC) of microglandular adenosis (MGA). IHC of MGA shows positive for S-100 protein (IHC stain, ×40). |
References
1. McDivitt RW, Stewart FW, Berg JW. Armed Forces Institute of Pathology (U.S.). Universities Associated for Research and Education in Pathology. Tumors of the breast. Atlas of Tumor Pathology, Second Serise, Fascicle 2. 1968. Wathington, D.C.: Armed Forces Institute of Pathology;91.
2. Rosen PP. Rosen's Breast Pathology. 2009. 3rd ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins;175–186.
3. Rosen PP. Microglandular adenosis. A benign lesion simulating invasive mammary carcinoma. Am J Surg Pathol. 1983. 7:137–144.
4. Geyer FC, Kushner YB, Lambros MB, Natrajan R, Mackay A, Tamber N, et al. Microglandular adenosis or microglandular adenoma? A molecular genetic analysis of a case associated with atypia and invasive carcinoma. Histopathology. 2009. 55:732–743.

5. Eusebi V, Foschini MP, Betts CM, Gherardi G, Millis RR, Bussolati G, et al. Microglandular adenosis, apocrine adenosis, and tubular carcinoma of the breast. An immunohistochemical comparison. Am J Surg Pathol. 1993. 17:99–109.

6. Popper HH, Gallagher JV, Ralph G, Lenard PD, Tavassoli FA. Breast carcinoma arising in microglandular adenosis: a tumor expressing S-100 immunoreactivity. Report of five cases. Breast J. 1996. 2:154–159.

7. Tavassoli FA, Bratthauer GL. Immunohistochemical profile and differential diagnosis of microglandular adenosis. Mod Pathol. 1993. 6:318–322.
8. James BA, Cranor ML, Rosen PP. Carcinoma of the breast arising in microglandular adenosis. Am J Clin Pathol. 1993. 100:507–513.

9. Shin SJ, Simpson PT, Da Silva L, Jayanthan J, Reid L, Lakhani SR, et al. Molecular evidence for progression of microglandular adenosis (MGA) to invasive carcinoma. Am J Surg Pathol. 2009. 33:496–504.

10. Diaz NM, McDivitt RW, Wick MR. Microglandular adenosis of the breast. An immunohistochemical comparison with tubular carcinoma. Arch Pathol Lab Med. 1991. 115:578–582.
11. Koenig C, Dadmanesh F, Bratthauer GL, Tavassoli FA. Carcinoma arising in microglandular adenosis: an immunohistochemical analysis of 20 intraepithelial and invasive neoplasms. Int J Surg Pathol. 2000. 8:303–315.

12. Acs G, Simpson JF, Bleiweiss IJ, Hugh J, Reynolds C, Olson S, et al. Microglandular adenosis with transition into adenoid cystic carcinoma of the breast. Am J Surg Pathol. 2003. 27:1052–1060.

13. Khalifeh IM, Albarracin C, Diaz LK, Symmans FW, Edgerton ME, Hwang RF, et al. Clinical, histopathologic, and immunohistochemical features of microglandular adenosis and transition into in situ and invasive carcinoma. Am J Surg Pathol. 2008. 32:544–552.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download