Abstract
Stereotactic vacuum-assisted breast biopsy (VAB) has been used to evaluate microcalcifications or non-palpable breast lesions on mammography. Although stereotactic VAB is usually performed in a prone or upright position, an expensive prone table is necessary and vasovagal reactions often occur during the procedure. For these reasons, the lateral decubitus position can be applied for stereotactic VAB, and true lateral mammography can be used to detect the lesion. We report on 15 cases of lateral decubitus positioning for stereotactic VAB with true lateral mammography for non-palpable breast lesions or microcalcifications. The mean procedure time was approximately 30.1 minutes, and no complications occurred during the procedures. Fourteen cases had benign breast lesions and one case had a ductal carcinoma in situ. The lateral decubitus stereotactic VAB with true lateral mammography can be applied for microcalcifications or non-palpable breast lesions and helps to minimize anxiety and vasovagal reactions in patients.
Microcalcified or non-palpable breast lesions represent 6-35% of beast malignancies [1,2]. However, it is difficult to target the exact site of a microcalcification or non-palpable breast lesion if it is not detectable by ultrasound. Because stereotactic vacuum-assisted breast biopsy (VAB) has a higher accuracy rate using a horizontal (x), vertical (y), and depth (z)-axis compared with ultrasound-guided VAB for breast microcalcification or non-palpable lesions, it has been used as a reliable technique to localize and sample those breast lesions [3,4]. Furthermore, if the targeting is successful, it is easier than ultrasound-guided VAB or surgical biopsy.
Stereotactic VAB is usually performed in a prone or upright position. However, an expensive, dedicated prone table is necessary to perform prone-positioned stereotactic VAB. Additionally, there are some limitations to upright stereotactic VAB, such as inconvenience or a vasovagal reaction of patients [2,5,6]. Although the lateral decubitus position is an alternative position for stereotactic VAB, the breast lesion often moves in the lateral decubitus position, as identified in cranio-caudal (CC) or mediolateral-oblique (MLO) mammography views [7]. Therefore, a true lateral mammography view is needed to overcome this problem, which is nearly identical to stereotactic mammography in a lateral decubitus position.
We used the lateral decubitus position during stereotactic VAB combined with true lateral mammography on 15 patients.
Between January and June 2010, we performed stereotactic directional VAB on 15 patients at the Breast Cancer Center of Pusan National University Hospital. In all cases, the CC and MLO mammography planes were preferentially reviewed to identify non-palpable lesions with or without microcalcifications, and those lesions were classified according to the Breast Imaging Reporting and Data System (BI-RADS). True lateral views of the mammographic images (GE Medical Systems, Milwaukee, USA) were obtained in all cases before lateral decubitus positioning for stereotactic VAB.
The patient laid on a stereotactic VAB table (Digital Stereotaxy with Senographe DS Interventional; GE Medical Systems) in the lateral decubitus position with the breast lesion facing up (Figure 1). The scout view and 15° paired stereotactic mammography were performed to localize the breast lesion, which was compared with a true lateral view (Figure 2). We calculated the horizontal (x) and vertical (y) distanced and the depth (z) from the zero point of the stereotactic VAB device. Local anesthesia was performed with 2 mL of a 2% lidocaine injection under aseptic conditions. The incision was usually 4 mm according to the skin crease. Stereotactic 15° paired mammographic views were obtained again after an 8-gauge VAB probe (Mammotome®; Ethicon-Endosurgery, Cincinnati, USA) was inserted to verify the site where the microcalcification was located. The needle position was corrected when the distance was more than 2 mm between the needle tip and the breast lesion.
Stereotactic VAB was performed by one clinical physician. Each of two breast tissue specimens were collected from six clockwise directions (2, 4, 6, 8, 10, and 12 o'clock) and arranged in a regular sequence on a Petri dish containing round filter paper (Figure 3). The stereotactic VAB was concluded when the breast lesion was identified by specimen mammography in more than two breast tissues. Additional sampling was performed when the breast lesion was still identified on stereotactic mammography or was not identified in specimen mammography. The procedure time was determined to be from probe insertion to removal.
A post-procedure mammography view was obtained after a breast marker clip (Johnson & Johnson, Ethicon-Endosurgery, Cincinnati, USA) was inserted. The breast was manually compressed at the procedure table. Only a piece of strip envelope (Steri-Strip; 3M, St. Paul, USA) and a compression bandage were used as a wound dressing.
Microcalcifications were observed in 12 (80.0%) of the 15 cases, and the remaining three cases (20.0%) showed non-palpable breast masses. Eight cases (53.3%) were classified as BI-RADS category 3 and seven (46.7%) were category 4 (Table 1). The morphological characters of the microcalcifications were scattered punctuate (n=5), clustered punctuate (n=4), amorphous (n=2) and pleomorphic (n=1). Non-palpable masses without microcalcifications were spiculated (n=2) and oval shape (n=1). Microcalcification clusters were 6-15 mm in diameter (mean, 10.3 mm) and masses were 6-8 mm in diameter (mean, 6.7 mm) (Table 2).
The mean patient age was 45.7 years (range, 33-63). Ten cases of microcalcifications were identified in the first specimen mammographic image, and the breast marker clip was located accurately in two cases of non-palpable masses after one procedure. The mean procedure time was approximately 30.1 minutes, and the average number and length of obtained core tissues was 14.4 (range, 9-26) and 2.7 cm (range, 0.8-3.3), respectively. No patient developed a vasovagal reaction such as nausea, vertigo, or syncope during the procedure. One week after the procedure, complications were identified by a physical examination and ultrasound. A minimal hematoma was observed in two cases (13.3%), which resolved spontaneously (Table 3).
In the histopathologic diagnoses of the stereotactic VAB, 14 cases had benign breast lesions and 1 case was a ductal carcinoma in situ (DCIS). Additional breast conserving surgery (quadrantectomy with a local flap) was performed for the malignant case. Among 12 cases of microcalcification on mammography, the microcalcification was confirmed in 11 (91.7%) by the pathological findings of normal stroma, benign ductules, fibrocystic changes, or DCIS. The other case of microcalcification on mammography revealed only benign ductules. Three cases without microcalcification on mammography were confirmed as sclerotic stroma with benign breast tissue. Fibroadenomas were identified in two cases, which were combined with fibrocystic changes and microcalcification (Table 4).
Stereotactic breast biopsy is a reliable procedure for localizing and sampling of breast tissue using mammography. Stereotactic VAB has a high sensitivity (98%) and specificity (100%) and may provide a promising alternative to open breast biopsy [1,8-12]. Among various type of stereotactic breast biopsy, stereotactic VAB is indicated for microcalcifications or non-palpable breast lesions not detectable by ultrasound. BI-RADS categories 3 or 4 for non-palpable breast lesions are usually applied for stereotactic breast biopsy, because category 5 or higher cases should be submitted to ultrasound-guided needle biopsy [13].
Stereotactic VAB is a very consistent and easy procedure if the needle is well located when targeting the breast lesion. Because accurate targeting is key for stereotactic VAB, the needle should be inserted at the exact site of the calculated x, y, and z-axis points from the zero point, which is set in the VAB machine. Furthermore, the complication rates for stereotactic VAB are relatively lower than ultrasound-guided VAB, because less breast tissue is collected. The failure rate for stereotactic VAB is 3-10%, mostly due to a difficult procedure site or a complication such as a lesion near the skin, muscle or axilla [2,4]. In our study, we did not encounter a case in which we could not use stereotactic VAB, and a surgical operation was added only for a malignant lesion. Because no further procedures were performed for the other 14 benign lesions, the sensitivity and specificity of stereotactic VAB could not be evaluated.
A limitation of stereotactic VAB is that less breast tissue volume is obtained than with ultrasound-guided VAB. The procedure completion time for stereotactic VAB is usually not indicated in studies and no definite guideline exists. We terminated the procedure when microcalcifications were seen in more than two tissues obtained for specimen mammography or when the breast marker clip was accurately located at a breast lesion on post-procedure mammography. Because stereotactic VAB focuses on diagnosis and not treatment, additional procedures are unnecessary. But, the rate of atypical ductal hyperplasia (ADH, 20.9%) or DCIS (11.2%) can be underestimated [8]. Therefore, identifying an ADH or DCIS during stereotactic VAB warrants a further procedure or surgical excision.
Although stereotactic VAB is definitely an innovative diagnostic procedure, its disadvantages are that it is conducted in a prone position and that the unit is very large and expensive (approximately quadruple that of a regular unit). It is also difficult to perform stereotactic VAB when the targeting site is close to the pectoralis muscle, axilla, or skin [6,13]. In contrast, stereotactic VAB in an upright position allows the breast lesion to be easily found with a scout image, which is almost same as the CC plane of original mammography. However, it is very difficult to maintain a stable position, because transient neck and back pain, as well as body numbness are common during the procedure, especially in the elderly. Moreover, patients can see the sharp devices, the wound and even blood, which cause anxiety, emotional stress, and possibly a vasovagal reaction, such as nausea, vertigo, or syncope [2,3,6].
To compensate for the disadvantages of the prone or upright-positioning for stereotactic VAB, a one-side decubitus position was used, which is more comfortable and stable for the patients, even if it has not been generally used [7]. An expensive prone table is unnecessary for lateral positioning during stereotactic VAB and it causes neither a vasovagal reaction nor neck, or back pain. But, it is more difficult to detect the breast lesion, because the lesions often moves, which is different from standard mammography. To alleviate this problem, we performed a true lateral mammography view before the procedure, which lengthened the procedure. Breast lesions could be easily identified on stereotactic mammography, because the targeted site was nearly the same as the pre-procedure true lateral view mammography.
Our report has limitations such as the small number of cases with a short term follow-up and the absence of a comparison between stereotactic VAB and other diagnostic procedures. According to several studies, 6-27% of benign breast lesions remain after a VAB procedure, and it is difficult to distinguish between a post-procedural scar and residual lesion within 6 months [14,15]. Therefore, long term follow-up is necessary to evaluate for a residual breast lesion. Although this report has simply introduced a diagnostic procedure, the usefulness of lateral decubitus positioning for stereotactic VAB with true lateral mammography could be verified if a comparison between stereotactic VAB and open biopsy or ultrasound-guided VAB with a long term follow-up result was added.
Figures and Tables
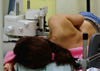 | Figure 1Patient laid on the stereotactic vacuum-assisted breast biopsy table in the lateral decubitus position with the breast lesion upside. |
 | Figure 2A scout 15° paired stereotactic mammography view. The targeted microcalcification is seen below the probe. |
 | Figure 3Specimen mammography shows a microcalcification in three separate specimens (yellow circles). |
Table 2
Characteristics of mammography in fifteen stereotactic vacuum-assisted breast biopsy (VAB) cases

References
2. Ohsumi S, Takashima S, Aogi K, Ishizaki M, Mandai K. Breast biopsy for mammographically detected non-palpable lesions using a vacuum-assisted biopsy device (Mammotome) and an upright-type stereotactic mammography unit. Jpn J Clin Oncol. 2001. 31:527–531.

3. Sim LS, Kei PL. Upright stereotactic vacuum-assisted needle biopsy of suspicious breast microcalcifications. J Med Imaging Radiat Oncol. 2008. 52:358–364.

4. Rovera F, Dionigi G, Marelli M, Ferrari A, Limonta G, Corben AD, et al. Breast cancer diagnosis: the role of stereotactic vacuum-assisted aspiration biopsy. Int J Surg. 2008. 6:Suppl 1. S104–S108.

5. Nisbet AP, Borthwick-Clarke A, Scott N. 11-gauge vacuum assisted directional biopsy of breast calcifications, using upright stereotactic guidance. Eur J Radiol. 2000. 36:144–146.

6. Wunderbaldinger P, Wolf G, Turetschek K, Helbich TH. Comparison of sitting versus prone position for stereotactic large-core breast biopsy in surgically proven lesions. AJR Am J Roentgenol. 2002. 178:1221–1225.

7. Welle GJ, Clark M, Loos S, Pauls D, Warden D, Sheffield M, et al. Stereotactic breast biopsy: recumbent biopsy using add-on upright equipment. AJR Am J Roentgenol. 2000. 175:59–63.
8. Yu YH, Liang C, Yuan XZ. Diagnostic value of vacuum-assisted breast biopsy for breast carcinoma: a meta-analysis and systematic review. Breast Cancer Res Treat. 2010. 120:469–479.

9. Kettritz U, Rotter K, Schreer I, Murauer M, Schulz-Wendtland R, Peter D, et al. Stereotactic vacuum-assisted breast biopsy in 2874 patients: a multicenter study. Cancer. 2004. 100:245–251.

10. Apesteguía L, Mellado M, Sáenz J, Cordero JL, Repáraz B, De Miguel C. Vacuum-assisted breast biopsy on digital stereotaxic table of nonpalpable lesions non-recognisable by ultrasonography. Eur Radiol. 2002. 12:638–645.

11. Choo KS, Kwak HS, Tae Bae Y, Lee JY, Lee SJ, Seo HI, et al. The value of a combination of wire localization and ultrasound-guided vacuum-assisted breast biopsy for clustered microcalcifications. Breast. 2008. 17:611–616.

12. Hung WK, Lam HS, Lau Y, Chan CM, Yip AW. Diagnostic accuracy of vacuum-assisted biopsy device for image-detected breast lesions. ANZ J Surg. 2001. 71:457–460.

13. Zagouri F, Sergentanis TN, Nonni A, Koulocheri D, Fotou M, Panopoulou E, et al. Vacuum-assisted breast biopsy: the value and limitations of cores with microcalcifications. Pathol Res Pract. 2007. 203:563–566.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download