Abstract
Purpose
Breast conservation surgery (BCS) has become a standard treatment method for patients with early breast cancer. Endoscopy-assisted BCS (EABCS) can be performed through an inconspicuous periareolar and a small axillary incision for sentinel node biopsy, which may give better cosmetic outcomes than conventional BCS skin incisions. This study was designed to evaluate the feasibility of EABCS for patients with early breast cancer.
Methods
Forty-three patients were candidates for EABCS, and EABCS was performed in 40 patients with breast cancer between January 2008 and July 2010. Their clinicopathological features were retrospectively analyzed. Operative time, margin status, complications, and relapse-free survival were compared with those of patients treated by conventional BCS and who were treated at the same institute during the same period.
Results
The most common lesion site of the EABCS and conventional BCS groups was the upper area of the breast. Tumor size in all patients was less than 4 cm (range, 0.4-3.7 cm), and nodal involvement was found in eight (20%) patients in the BCS group. The mean operative time was 110 minutes for the EABCS group and 107 minutes for the conventional BCS group, and those were not significantly different. No significant difference in frozen or final margin status was observed between the EABCS and conventional BCS groups. Relapse-free survival was statistically equivalent between the groups with a median follow-up of 12 months. Postoperative complications occurred in five cases in four patients with EABCS, which was not significantly different from conventional BCS.
Breast conservation surgery (BCS) is a standard procedure for patients with breast cancer, and BCS followed by radiation provides better cosmetic outcomes with acceptable oncological safety than mastectomy [1]. A long marked scar have been a issue in some patients with small-sized breasts; thus, endoscopy-assisted BCS (EABCS) for patients with early breast cancer has been introduced mainly from Asian countries including Korea and Japan because Asian women tend to have smaller breasts than those of Western women [2-6].
The main advantage of EABCS is an inconspicuous scar, because it is performed via a small periareolar and axillary incision for the sentinel lymph node biopsy as an endoscopy port. For this reason, endoscopy-assisted breast surgery has been successfully established in the field of aesthetic and plastic surgery [7-9]. The feasibility and oncological safety of EABCS has been shown in short-term results [6,10,11]. Fukuma [3] suggested that the curability of endoscopic breast surgery including EABCS is the same as that with conventional methods and the local recurrence rate after a total and partial mastectomy.
However, most studies regarding EABCS are small-sized retrospective cross-sectional studies, and only a few studies are case-controlled trials comparing endoscopic surgery with conventional surgery [10,12]. The current study was conducted to investigate the feasibility of EABCS in patients with early breast cancer by comparing EABCS and conventional BCS in terms of operative time, resection margin status, complication rate, and relapse-free survival (RFS).
Forty-three patients with breast cancer were candidates for EABCS at Severance Hospital, Yonsei University Health System between January 2008 and July 2010. We excluded three patients who converted to a mastectomy; one was due to a very poor breast shape due to insufficient volume of the remaining breast, and two were due to positive resection margins on frozen biopsy. Thus, 40 patients were finally included in the analysis (Figure 1). All EABCS patients with breast cancer met the following criteria at the initial diagnosis: clinical tumor size less than 5 cm, clinically node negative, and no evidence of invasion to the skin, pectoralis muscle, or chest wall. All of these criteria were evaluated using mammography, ultrasonography, and/or magnetic resonance imaging. The patient medical records were retrospectively reviewed. A total of 766 patients underwent conventional BCS at the same institute during the same period, and 85 patients among them were mastectomy conversion cases due to positive resection margins on frozen pathology specimens. Conventional BCS was performed in patients with early breast cancer who had tumors less than 5 cm, and we excluded patients treated with neoadjuvant chemotherapy. We finally reviewed the medical data of 681 patients who underwent conventional BCS. Frozen margin status and operative time were recorded in an electric medical record (EMR) and were reviewed using the clinical data repository system of the Yonsei University Health System. We excluded cooperative cases, such as a thyroidectomy performed with BCS at the same time, when calculating the mean total operative time. RFS was defined as the time from the date of operation to the date of the first event. RFS events were loco-regional relapse, systemic relapse, and death from any cause. Postoperative EABCS complications recorded in the EMR system were also reviewed. Tumor stages were determined based on the 7th American Joint Committee on Cancer criteria. All patients who underwent EABCS or conventional BCS were scheduled to receive radiation therapy. Estrogen receptor (ER) and progesterone receptor (PR) status of primary breast cancer were evaluated from formalin-fixed, paraffin-embedded whole sections of surgically resected breast cancer specimens using immunohistochemistry (IHC). Primary antibodies for ER (clone SP1; NeoMarkers for Lab Vision, Fremont, USA), and PR (clone PgR 636; DAKO, Glostrup, Denmark) were used for the IHC staining.
Comparisons between continuous variables were analyzed with the Student's t-test. Comparisons between categorical variables were assessed using the Pearson's chi-square test or the Fisher's exact test. All tests were two sided. Survival curves were plotted and estimated using the Kaplan-Meier method. Intergroup differences in the survival time were accessed by the log-rank test. A p-value <0.05 was statistically significant. PASW 18 (SPSS Inc., Chicago, USA) was used for all statistical analyses.
The EABCS surgical technique is described briefly as follows (Figure 2). A patient was laid in the supine position in the same manner as for the conventional BCS procedure under general anesthesia. After sterile surgical draping, a surgeon identified the location of the tumor, and a sentinel lymph node biopsy was performed via a 3-cm ipsilateral axillary incision. We performed the sentinel lymph node biopsy using technitium-99m in the same manner as a routine sentinel lymph node biopsy, and did not use endoscopic devices (Figure 2A). The dissected sentinel lymph nodes were processed immediately for frozen biopsy, and then the surgeon directly identified the lateral border of the pectoralis muscle using Mezenbaum scissors or a conventional Bovie electrocauterizer through the axillary incision. If the frozen biopsy revealed a metastasis, a conventional level I and II axillary lymph node dissection was performed via an extended axillary incision. After the dissection had been carried deep over the lateral edge of the pectoralis major muscle, the retromammary space was identified and dissected using an Endosector LE (Curexo, Anyang, Korea), which was introduced in a previous study [6], under the view of an endoscopic video system (Figures 2B, 3A). The endoscopy was 5-mm in diameter, rigid, and oblique at 30°. The retromammary space dissection should be performed with caution to avoid dissecting the interpectoral space. Sufficiently dissecting the retromammary space of the quadrant to be resected at the beginning of the quadrantectomy is important because it mobilizes the remaining breast parenchyma and eases the resection and re-modeling of the remaining tissue (Figure 2C). A surgical gauze pad was placed in the retromammary space to avoid iatrogenic injury to the pectoralis major muscle. A 3-4 cm periareolar incision was made after marking the resection margin using a gentian-violet dye injection along the margin 1 cm distant from the tumor (Figure 2D). Subcutaneous flaps were constructed using an electrocauterizer with conventional retractors. We first dissected the closest margin to the nipple-areolar complex for the frozen biopsy to exclude cancer invasion into this area, and the lump containing the lesion was excised using a knife (Figure 2E). The gauze placed in the retromammary space was removed, and the cavity margins were resected and processed for a frozen biopsy. Surgical clips were inserted to inform radiation oncologists of the exact location of the main lesion (Figure 3B). We inserted a suction drain if necessary, and the adjacent breast parenchyma was mobilized and approximated with minimal distortion to the remaining breast. We sealed the wound with two layers of gauze after skin closure (Figure 2F). All specimens obtained from the operation were processed for routine pathology. The postoperative images of the patients treated with EABCS and conventional BCS are shown in Figure 4.
The median age of patients treated with EABCS was 51 years (range, 28-68 years), and all patients were female. No differences in the clinicopathological characteristics were observed, except T-stage; and mean tumor size was not different between the two groups (Table 1). Most lesions in the EABCS group were located in the upper medial or lateral quadrant of the breast, and no subareolar or multifocal lesions were observed in patients treated with EABCS. No significant difference was found in the positive margin status rates of the initial frozen biopsy between the EABCS and conventional BCS candidates (Table 2).
Margin positive cases were reported in two patients in the EABCS group and in 11 patients in the conventional BCS group (Table 2). Two patients revealed a positive margin for the permanent pathology. Those cases were all negative in frozen sections during the operation, and focal lobular carcinoma in situ and ductal carcinoma in situ margins were revealed in the permanent pathology. These cancers were treated by whole breast radiation with 9-14 Gy boost radiation therapy on the tumor bed.
The mean total operative time for BCS tended to be slightly longer than that of conventional BCS; however, the difference was not statistically significant (Table 3). Axillary lymph node dissection due to a positive result on the sentinel lymph node biopsy prolonged mean total operation time by 37 minutes in the EABCS group and by 20 minutes in the conventional BCS group; however, the difference was not statistically significant between the two groups.
Postoperative complications occurred in five cases of four patients in the EABCS group; two of five cases had prolonged seroma with inflammation, one had cellulitis, one developed skin wound necrosis, and one had postoperative bleeding (Table 4). These patients were managed with conservative treatment. The difference in the complication rates between the EABCS and conventional BCS groups was not statistically significant.
No recurrences were reported in the EABCS group and two recurrences occurred in the conventional BCS group with a median follow-up of 12 months (Figure 5). No significant difference in RFS was observed between the two groups.
The advantage of EABCS is an inconspicuous scar, which may increase a patient's satisfaction [5,6,10]. In addition, early dissection of the retromammary space with endoscopic devices before forming the subcutaneous flap may help with the handling of the breast tissue to be resected and make remodeling of the remaining breast tissues easier. In contrast, the additional cost for endoscopic devices and the learning period to develop the EABCS technique are a disadvantage of EABCS. Although the Japanese health authority agreed to reimburse the cost for endoscopic breast surgery, no definite reimbursement consensus was reached for endoscopic breast surgery in Europe and North America [11]. Compared with previous techniques performed with various endoscopic devices, retractors, or enhanced coagulating and cutting devices such as the harmonic scalpel [5,6,13], our technique can be performed with only one set of endoscopic devices including an Endosector LE and an endoscopic video system for dissecting the retromammary space. Therefore, the cost for using the additional devices might be reduced. Furthermore, inserting surgical clips into the space may help inform the radiation oncologists about the exact resection area for appropriate radiation therapy.
The feasibility of EABCS was analyzed in terms of operative time, resection margin status, and RFS. Operative time is usually affected by the number of cumulative cases or operation type [5,10], and it has been reported that EABCS takes longer than conventional BCS [6]. However, although we included EABCS cases during the earlier period, which usually took longer than those after the learning period, to calculate the mean operative time, the difference in the mean operative time between EABCS and conventional BCS was only about 10 minutes, and it was not significant. This finding was correlated with that of a previous study [10].
Resection margin status is a risk factor for local recurrence in patients with early breast cancer [14,15], and it may be a crucial factor for accessing EABCS feasibility. The resection margin positive rate for EABCS is 0-5% [5,6,10], compared with about a 10% margin positive rate for conventional BCS [1,16]. A direct comparison of the differences in the positive margin rates between EABCS and conventional BCS is difficult due to heterogeneous study designs, including the definition of a positive margin, surgeon experience, and extent of surgery. The current study demonstrated no significant difference in resection margin status, including frozen and permanent pathological results, between EABCS and conventional BCS, although all procedures were performed at a single institution and included patients treated by EABCS during the early learning period.
Only a few studies have reported recurrence or survival data for EABCS. Previous studies indicated that patients with EABCS have no local or distant recurrence with a mean follow-up period of 24-25 months, and the retrospective data of 966 patients with EABCS from a single institute in Japan reported a 0.62% local recurrence rate [6,10,11]. These results correspond with our data. However, those studies, including our study, were retrospective trials, and the follow-up periods were short. Further randomized controlled trials with a longer-term follow-up should be conducted to support this technique.
The EABCS complications seemed to be acceptable. Hemorrhage, hematoma, skin burns, and wound infections are common complications of EABCS in the literature, and those complications are treatable and the possibility of re-operation due to the complications is low [6,10]. Our study showed similar results to previous studies, and we also demonstrated that the complication rates in the EABCS group were not significantly different from those in the conventional BCS group.
Complete mastery of EABCS requires eye-hand coordination, a basic understanding of endoscopic instruments, and adequate patient selection. This may be the key to success of endoscopic breast surgery, and learning from other endoscopic surgery may help to enhance EABCS skill [11].
Nonetheless, there are some limitations to our study. This study was a retrospective trial with a small volume of enrolled patients and a short follow-up period; therefore, further prospective trials with a longer-term follow-up will be necessary to confirm the clinical role of EABCS. However, to our knowledge, this is the only study that has compared EABCS with conventional BCS in terms of various clinicopathological features such as operative time, complication rates, margin status, and RFS.
In conclusion, the results show that operative time, complications, resection margin status, and RFS of EABCS in patients with early breast cancer were not significantly different from those of conventional BCS. The complication rate of EABCS was not serious and the complications were treatable. Some novel EABCS approaches including the minimal use of endoscopic devices and inserting a surgical clip have been introduced. Thus, although the oncological safety has not yet been confirmed, this study indicated that EABCS for patients with early breast cancer is feasible and safe. Taken together, EABCS may play a role in better cosmetic scar management for patients with early breast cancer.
Figures and Tables
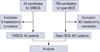 | Figure 1Study enrollment scheme.
BCS=breast conservation surgery; EABCS=endoscopy-assisted breast conservation surgery.
|
 | Figure 2Illustrations of the surgical technique. (A) Sentinel lymph node biopsy using technitium-99m was performed in the same manner as a routine sentinel lymph node biopsy. (B) The dissection was carried deep over the lateral edge of the pectoralis major muscle, and then the retromammary space was identified and dissected using Endosector LE under the view of an endoscopic video system. (C) Sufficient dissection of the retromammary space of the quadrant to be resected at the beginning of the quadrantectomy is important because it can mobilize the remaining breast parenchyma and provides easy handling of the resection and re-modeling of the remaining tissue. (D) The resection margin was marked using an injection of gentian-violet dye along the margin 1 cm distant from the tumor. (E) The lump containing the main lesion was excised using a knife after making a periareolar incision and constructing the subcutaneous flaps. Note that surgical clips were applied at the margins to inform the radiation oncologist of the exact location of the main lesion. (F) The breast parenchyma and skin wound were approximated. |
 | Figure 3Endoscopic findings of the retromammary space and insertion of surgical clips. (A) Note that the Endosector LE was placed just above the pectoralis major muscle and was slid over the retromammary space. (B) Arrow indicates a surgical clip in the remaining breast parenchyma. We usually inserted the surgical clips in four directions: nipple, inner and/or outer, and upper and/or lower areas of margins. |
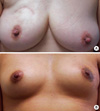 | Figure 4Postoperative scars from endoscopic-assisted breast conservation surgery and conventional breast conservation surgery. (A) Postoperative scar after conventional breast conservation surgery (BCS). (B) Postoperative scar after endoscopic-assisted breast conservation surgery (EABCS). Note that the periareolar scar of the patient who underwent EABCS is more inconspicuous than the incision scar of the patient who underwent conventional BCS. |
 | Figure 5Relapse-free survival (RFS) between endoscopic-assisted breast conservation surgery (EABCS) and conventional breast conservation surgery (BCS). |
ACKNOWLEDGMENTS
The authors are grateful to Dong-Su Jang (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the illustrations.
References
1. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002. 347:1233–1241.

2. Ingram D. Is it time for breast cancer surgeons to embrace endoscopic-assisted mastectomy? ANZ J Surg. 2008. 78:837–838.

3. Fukuma E. Endoscopic breast surgery for breast cancer. Nippon Geka Gakkai Zasshi. 2006. 107:64–68.
4. Tamaki Y, Miyoshi Y, Noguchi S. Application of endoscopic surgery for breast cancer treatment. Nippon Geka Gakkai Zasshi. 2002. 103:835–838.
5. Lee EK, Kook SH, Park YL, Bae WG. Endoscopy-assisted breast-conserving surgery for early breast cancer. World J Surg. 2006. 30:957–964.

6. Hong YI, Shin H. Endoscopy-assisted breast conserving surgery for breast cancer: a preliminary clinical experience. J Breast Cancer. 2010. 13:138–146.

7. Giordano PA, Rouif M, Laurent B, Mateu J. Endoscopic transaxillary breast augmentation: clinical evaluation of a series of 306 patients over a 9-year period. Aesthet Surg J. 2007. 27:47–54.

8. Eaves FF 3rd, Bostwick J 3rd, Nahai F, Murray DR, Styblo TM, Carlson GW. Endoscopic techniques in aesthetic breast surgery. Augmentation, mastectomy, biopsy, capsulotomy, capsulorrhaphy, reduction, mastopexy, and reconstructive techniques. Clin Plast Surg. 1995. 22:683–695.
10. Yamashita K, Shimizu K. Endoscopic video-assisted breast surgery: procedures and short-term results. J Nippon Med Sch. 2006. 73:193–202.

11. Keshtgar MR, Fukuma E. Endoscopic mastectomy: what does the future hold? Womens Health (Lond Engl). 2009. 5:107–109.

12. Kitamura K, Ishida M, Inoue H, Kinoshita J, Hashizume M, Sugimachi K. Early results of an endoscope-assisted subcutaneous mastectomy and reconstruction for breast cancer. Surgery. 2002. 131:1 Suppl. S324–S329.

13. Owaki T, Yoshinaka H, Ehi K, Kijima Y, Uenosono Y, Shirao K, et al. Endoscopic quadrantectomy for breast cancer with sentinel lymph node navigation via a small axillary incision. Breast. 2005. 14:57–60.

14. Nishimura R, Akizuki M, Tashima R, Ootao R. Investigation of factors related to periods to ipsilateral breast tumor recurrence after breast-conserving surgery and measures for preventing recurrence in early breast cancer. Breast Cancer. 2006. 13:152–158.

15. Dunne C, Burke JP, Morrow M, Kell MR. Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009. 27:1615–1620.

16. Freedman G, Fowble B, Hanlon A, Nicolaou N, Fein D, Hoffman J, et al. Patients with early stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int J Radiat Oncol Biol Phys. 1999. 44:1005–1015.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download