Abstract
Purpose
To study clinical features and patterns of recurrence after breast-conserving treatment (BCT) for three molecular subtypes of early stage breast cancer.
Methods
The sample studied included 596 patients with T1-2N0-1 breast cancer who received BCT. Three groups were defined by receptor status. Luminal: estrogen receptor (ER) or progesterone receptor (PR) positive; triple negative (TN): ER, PR, and epidermal growth factor receptor-2 (HER2) receptor negative; and HER2 overexpressing: ER and PR negative but HER2 receptor positive.
Results
The number of patients in each group was 408 (68.5%), 105 (17.6%), and 83 (13.9%), respectively. The median follow-up period was 79 months. The TN and HER2 subtypes occurred in younger patients (p=0.0007) and had higher nuclear grade and poorer histologic grade (p<0.0001 and 0.0071, respectively). During the follow-up period, locoregional recurrence was detected as the first site of recurrence in 26 (6.4%), 11 (10.5%), and 9 (10.8%) patients in the luminal, TN, and HER2 subtypes, respectively (p=0.1924). Thirty-one (7.6%), 7 (6.7%), and 7 (8.4%) patients in each group had distant metastases as the first sign of recurrence (p=0.8996). Median time to locoregional and distant recurrence was shorter in the HER2 subtype (p=0.0889 and 0.0780, respectively), and the HER2 subtype was significantly associated with poor overall survival (p=0.0009).
Breast cancer encompasses a heterogeneous population of tumors characterized by a variety of clinical, pathological, and molecular features [1-3]. Molecular markers such as estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) have been used to classify patients into different subtypes. Recently, gene expression studies using DNA microarrays have identified five common subtypes of breast cancer: luminal A (ER or PR positive and HER2 negative), luminal B (ER or PR positive and HER2 positive), HER2 overexpressing (ER and PR negative and HER2 positive), basal-like (ER, PR, and HER2 negative, CK 5/6 positive) and normal breast-like tumor [2,4-6]. HER2 overexpressing and basal-like subtypes are significantly more likely to be grade 3 and are associated with worse recurrence rates and decreased overall survival [3,5-7]. The triple negative (TN) phenotype, which includes tumors that do not express ER, PR, or HER2 on immunohistochemistry (IHC), serves as a proxy for the basal-like subtype with a positive predictive value of approximately 80-97% [8,9]. Although this approach is not complete, several groups have adopted a TN definition for basal-like cancer out of convenience.
Several randomized trials have demonstrated that survival rates following breast-conserving treatment (BCT) were equivalent to those observed with radical mastectomy [10-12]. Given these results, BCT has become an accepted treatment for early stage breast cancer [13]. At the present time, there is little clinical data evaluating whether a particular breast cancer subtype is associated with increased rates of local and/or distant recurrence after BCT [14-17].
The purpose of this study is to determine whether any particular breast cancer subtype, as approximated by ER, PR, and HER2, is associated with patterns of recurrence among women with early stage breast cancer who receive BCT.
Between September 1994 and December 2002, 1,537 patients with pathologic stage I-III breast cancer according to the American Joint Committee on Cancer stages were treated with curative surgery without neoadjuvant chemotherapy at Samsung Medical Center. All patients had available ER, PR, and HER2 receptor status. Medical records were retrospectively reviewed to find patients with pathologic T1-2N0-1 stage who received BCT. The number of eligible patients was 596.
Patients were classified according to the receptor status: luminal (ER or PR positive), TN (ER, PR, and HER2 negative), and HER2 (ER, PR negative, and HER2 positive). ER and PR status was determined on the basis of IHC staining. Positive HER2 was determined using either IHC 3+ staining or amplification on fluorescence in situ hybridization (FISH).
The median follow-up duration, calculated from the date of surgery, was 79 months (range, 5 to 147 months). ANOVA and chi-square tests were used to compare the differences in patient characteristics among the three groups. The endpoints of the study were locoregional or distant recurrence as the first event, overall survival (OS), and disease-free survival (DFS). OS was defined as the time from the date of surgery to death from any cause, and DFS was defined as the time from the date of surgery to relapse or death. Kaplan-Meier analyses for OS and DFS were estimated for each group and compared using the log-rank test. Cox proportional hazards regression analysis was used for multivariate analysis. The SAS software (SAS 9.0; SAS Institute Inc., Cary, USA) was used for statistical analysis. A probability value of less than 0.05 was considered statistically significant.
Among the 596 eligible patients, 391 (65.6%), 298 (50.0%), and 297 (49.8%) patients showed positive immunoreactivity for ER, PR, and HER2, respectively. As a result, 408 (68.5%), 105 (17.6%), and 83 (13.9%) patients were classified into the luminal, TN, and HER2 overexpressing groups, respectively. The characteristics of the patients in each subgroup are outlined in Table 1. The TN and HER2 subtypes occurred in younger patients (p=0.0007) and were more frequently of a higher nuclear grade and poorer histologic grade (p<0.0001 and 0.0071, respectively). There were also significant differences in the distribution of T stage (p=0.0181) and the extensive intraductal component (p=0.0119). The distribution of N stage, the overall pathologic stage, lymphovascular invasion (LVI), and resection margin was not different between the three subtypes.
All eligible patients underwent BCT and postoperative whole breast radiotherapy. The most common dose delivered was 50.4 Gy in 28 fractions (555 patients, 93.1%). The primary tumor bed was boosted in 538 (90.3%) patients with the most common dose being 10 Gy in 5 fractions (472 patients, 87.7%). A separate supraclavicular or axillary field was not added. Adjuvant systemic chemotherapy was delivered to 417 (70.0%) patients and the most common regimen was a combination of cyclophosphamide, methotrexate, and 5-fluorouracil (357 patients, 85.6%, Table 2). Among ER or PR positive patients, 82.3% (336 of 408) received hormonal therapy (Table 2). No patients received adjuvant trastuzumab therapy.
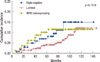
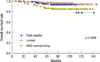
During the follow-up period, there were 26 (6.4%), 11 (10.5%), and 9 (10.8%) patients in each group who experienced loco-regional recurrence as the first site of recurrence (p=0.1924) (Table 3). The 5-year cumulative incidence of locoregional recurrence was 4.1%, 7.0%, and 10.1%, respectively (p=0.1510) (Figure 1). Median time to locoregional recurrence was 50.5, 40, and 28 months in each group, respectively (p=0.0889). The local area of the original tumor was the most frequent site of locoregional recurrence among the luminal (18 patients, 69.2%) and HER2 (8 patients, 88.9%) subtypes. Among the patients in the TN group, the supraclavicular area was the most frequent site (6 patients, 54.5%). Distant metastasis as the first site of recurrence occurred in 31 (7.6%) patients with the luminal subtype after a median time of 38 months. The median time to distant recurrence of TN and HER2 subtype was 26 and 21 months, respectively (p=0.0780). The overall incidence of distant recurrence during the follow-up period was 6.7% and 8.4% in the TN and HER2 subtypes, respectively (p=0.8996). The 5-years cumulative incidence of distant recurrence was 6.0%, 5.9%, and 8.6%, respectively (p=0.9348)(Figure 2).
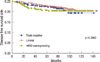
The 5-years OS and DFS rates of all patients were 95.4% and 89.7%, respectively. OS of the HER2 subtype was significantly poorer than other subtypes in univariate analysis (p=0.0009). The 5-year OS rate of each subtype was 97.4%, 94.9%, and 86.4%, respectively (Figure 3). DFS was not significantly different between the three subtypes (p=0.3862)(Figure 4). By multivariate Cox proportional hazard models, tumor subtype, nuclear grade, and histologic grade were significant prognostic factors affecting OS (Table 4). The hazard ratio of the TN and HER2 subtypes were 1.63 (95% confidence interval [CI], 0.46-5.73; p=0.4461) and 2.43 (95% CI, 1.44-4.09; p=0.0009), respectively. LVI, nuclear and histologic grade significantly affected DFS.
In this series, the recurrence patterns of 596 patients with conservatively-treated early breast cancer were evaluated based on ER, PR, and HER2 expression status. Although negative expression for the three markers has previously been used to categorize tumors such as basal-like cancer, the incompleteness of the definition is a potential weakness of this study [1,3-6]. This study was also limited due to its retrospective analysis, relatively small sample size in the HER2 and TN subgroups, and potential existence of other variables. Because no patient received trastuzumab therapy, the results could be changed by trastuzumab therapy. During the follow-up period, overall incidence of locoregional and distant recurrence as first site of recurrence was not significantly different between subgroups. Although not statistically significant, the time to recurrence in the HER2 subtype was shorter than that of other subtypes.
Nguyen et al. [15] reported a series of 793 patients with invasive breast cancer who received BCT. Based on the three markers, patients were categorized into four groups: luminal A and B, basal, and HER2. The basal and HER2 subtypes were associated with younger patients, higher grade tumors, and increased risk of local recurrence. The median age of the TN and HER2 subtypes was 51 and 49 years, respectively. The two subtypes were also associated with an increased risk of distant metastases than seen in the luminal A subtype. In this series, the TN and HER2 subtypes were also associated younger age and higher grade. But there were two differences between the two series. First, the median age of the TN and HER2 subtypes in the current series was 42 and 43 years, respectively, which was relatively younger than that of the other series. Second, an increased risk of local or distant recurrence was not seen in the present study. But the 5-year cumulative incidence of locoregional recurrence in luminal subgroup was 4.1%, which was relatively higher than that of the luminal A and B group in the other series (0.8% and 1.5%, respectively). The incidence of the TN and HER2 groups did not differ much from each other (7.0% and 10.1% vs. 7.1% and 8.4%, respectively).
Two additional studies showed that the TN or HER2 subtypes do not appear to be at a significantly increased risk for local or locoregional recurrence [16,17]. In the series of Dent et al. [16], the mean age was significantly younger for the TN group compared with the other group (53.0 vs. 57.7 years, p<0.0001). Also, patients in the TN group were more likely to have grade III tumors (66% vs. 28%, p<0.0001) that were larger. Despite such different characteristics, the TN subtype had a similar overall local recurrence rate to that of other subtypes of breast cancer (13% vs. 12%, p=0.77), but the mean time to local recurrence was shorter in patients with the TN subtype (2.8 vs. 4.2 years, p=0.02). The median time to distant recurrence was also shorter in the TN group (2.6 vs. 5.0 years, p<0.0001) and the proportion of patients with distant recurrence was higher in the TN subtype (33.9% vs. 20.4%, p<0.0001). The trend of shorter disease-free interval could be found in the HER2 subtype of our series, although the p-value was not statistically significant.
Freedman et al. [17] defined three groups similar to the current series: ER or PR (+), HER2, and TN. As was seen in the present study, patients in the TN and HER2 groups were more likely to have T2 and grade 3 diseases. The median age of the TN and HER2 groups was both 54 years, which was also older than that of the present study. They also reported that there was no overall difference in total locoregional recurrence rates between the three groups (p=0.13). Additionally, a higher rate of distant metastases in the HER2 group was observed (11.9%, p=0.009) which translated into a lower recurrence-free survival rate (84%, p=0.005).
The major difference between the current series and those reported previously is that there were no significant differences in overall incidence of distant metastases between the three groups during the follow-up period. Because the first site of recurrence was selected as an end point and included early stage patients who received BCT into analysis, the incidence of distant metastases could increase by the addition of patients who experienced local recurrence first followed by distant recurrence and who had more advanced disease. DFS was not significantly different between the three subtypes, but OS rate of the HER2 group was decreased compared to that of the other groups (p=0.0009).
In addition, the age distribution of the current series was relatively younger than that of the others. It has been known that the age distribution of breast cancer incidence in Korea is different from that in the United States and in other Western countries [18,19]. The peak age of incidence is younger in Korea, which was also shown in this series. Despite the retrospective nature with relatively small number of patients, the recurrent patterns after BCT according to molecular subtype in Korean patients with relatively younger age are remarkable feature of this study.
Having the HER2 subtype of tumor seems to carry a worse prognosis; however, the use of adjuvant trastuzumab, a humanized monoclonal antibody directed against HER2, could improve the clinical outcomes in the HER2 overexpressing subtype [20,21]. Two large randomized trials reported the benefit of adjuvant trastuzumab. In the Herceptin Adjuvant (HERA) trial, the unadjusted hazard ratio for the risk of an event in the trastuzumab group was 0.54 (95% CI, 0.43-0.67; p<0.0001), which corresponded to an absolute benefit of 8.4% in disease-free survival at 2 years [20]. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 trial and the North Central Cancer Treatment Group (NCCTG) N9831 trial showed a hazard ratio of 0.48 for a first event in the trastuzumab group (95% CI, 0.39-0.59, p<0.0001). The absolute benefit in DFS was 11.8% at 2 years [21].
Because of their lack of receptors for ER, PR, and HER2, those patients with TN breast cancer are not candidates for adjuvant hormonal therapy or trastuzumab. However, they may be candidates for targeted antibodies against epidermal growth factor receptor (EGFR) because basal-like tumors have been shown to overexpress EGFR [8,22,23]. Future studies are needed to establish the role of novel targeted treatments in patients with TN and HER2 breast cancer.
In summary, the TN and HER2 breast cancer occurred in younger patients and were more likely to have high grade tumors. The HER2 subtype was significantly associated with poor OS, but there was no difference in patterns of recurrence according to molecular subtypes among women with early stage breast cancer who had received BCT.
Figures and Tables
References
1. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000. 406:747–752.

2. Sørlie T. Molecular portraits of breast cancer: tumour subtypes as distinct disease entities. Eur J Cancer. 2004. 40:2667–2675.

3. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001. 98:10869–10874.

4. Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005. 23:7350–7360.

5. Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003. 100:8418–8423.

6. Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003. 100:10393–10398.

7. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006. 295:2492–2502.

8. Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004. 10:5367–5374.

9. Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008. 26:2568–2581.

10. van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000. 92:1143–1150.

11. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002. 347:1227–1232.

12. Poggi MM, Danforth DN, Sciuto LC, Smith SL, Steinberg SM, Liewehr DJ, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003. 98:697–702.

13. Lazovich D, Solomon CC, Thomas DB, Moe RE, White E. Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus Development Conference on the treatment of patients with early stage invasive breast carcinoma. Cancer. 1999. 86:628–637.

14. Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006. 24:5652–5657.

15. Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008. 26:2373–2378.

16. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007. 13(15 Pt 1):4429–4434.

17. Freedman GM, Anderson PR, Li T, Nicolaou N. Locoregional recurrence of triple-negative breast cancer after breast-conserving surgery and radiation. Cancer. 2009. 115:946–951.

18. Korean Breast Cancer Society. Clinical characteristics of breast cancer patients in Korea in year 2000. J Korean Breast Cancer Soc. 2002. 5:217–224.
19. Yoo KY, Shin A. Epidemiological characteristics of breast cancer in Korea. J Korean Breast Cancer Soc. 2002. 5:209–211.

20. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005. 353:1659–1672.
21. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005. 353:1673–1684.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download