Abstract
Sjögren's syndrome (SS) is an autoimmune disease that chronic inflammation and lymph node proliferation. Patients with SS carry a greater risk of developing lymphoproliferative malignancy. In addition to other organ cancers, breast cancer may also occur in these patients. Considering these, breast cancer in patients with SS can be misdiagnosed as being in an advanced stage particularly in the presence of axillary lymphadenopathy. Here, we report a rare case of a 45-year-old woman with SS who presented with a breast mass. Radiology showed a 4 cm solid lesion and conglomerates of axillary lymphadonepathy. A breast biopsy revealed ductal carcinoma in situ. A modified radical mastectomy was performed; however, no axillary metastases were detected. Clinicians should remain vigilant to the possibility that a false clinical impression of axillary metastasis may occur in such patients with breast cancer. Therefore, axillary node status should be verified first.
Sjögren's syndrome (SS) is a chronic inflammatory autoimmune disease characterized by progressive lymphocytic infiltration of the exocrine glands and other extra-glandular structures [1]. Dryness of the eyes (xerophthalmia leading to keratoconjunctivitis sicca) and mouth (xerostomia) constitute the typical clinical condition of this syndrome [1,2]. SS may also cause chronic multiple lymphadenopathy due to its lymphoproliferative characteristics. Although patients with SS have a lifespan not significantly different from that of the normal population, these patients carry a greater risk of developing lymphoproliferative malignancy [3]. In addition, other organ malignancies can also occur in patients with SS such as lung, breast, gastrointestinal, gynecological, renal, and skin cancers [3,4]. Considering all these, the diagnosis of breast cancer in patients with SS can be mistaken as cancer at an advanced stage particularly in the presence of axillary multiple lymph nodes.
In this study, we report a very rare case of breast cancer associated with SS in which the cancer was overstaged and, thus, overtreated on the assumption of clinically positive axillary lymph node status.
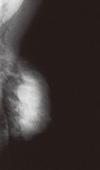
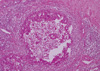
A 45-year-old woman with a 12-year history of SS presented with a complaint of a breast mass. A breast examination revealed a palpable mass, measuring 10 cm in diameter, located in the upper lateral quadrant of the right breast and right axillary multiple lymph nodes. On ultrasonography, the mass was a 4 cm solid lesion located in heterogeneous breast parenchyma, and conglomerates of multiple axillary lymphadenopathy suspicious of malignancy were also detected (Figure 1). The mammography was almost normal due to the dense parenchymal pattern, except for the presence of the axillary lymphadenopathy (Figure 2). A core-needle biopsy was performed on the breast lesion, and the diagnosis of ductal carcinoma in situ (DCIS) was made preoperatively. Based on the clinical suspicion of an advanced-stage breast cancer, the patient underwent a modified radical mastectomy. On postoperative histopathological assessment, the diagnosis of 8-cm high-grade DCIS was established, however no axillary lymph node metastases were detected (Figure 3).
SS is an autoimmune disease that causes chronic inflammation and primarily affects the exocrine glands [1,2]. Because it is a systemic disease, SS can also exhibit a wide range of clinical manifestations. Among these, benign or malignant lymphoproliferation due to infiltration by lymphocytes and plasma cells may be a prominent part of this syndrome [5]. Several studies have shown a high incidence of malignancy in SS, the most common being lymphoma [3,4]. Other non-lymphoid cancers including breast cancer can also occur in patients with this syndrome; however, an increase in the incidence of these cancers has not been demonstrated. Lazarus et al. [4] reported on a retrospective cohort study of 112 patients with SS in whom 25 developed malignancies, including three cases of breast cancer. In a recent study reported by Zhang et al. [3], malignancy was detected in 29 (2.2%) of 1,320 patients with SS, including four patients with breast cancer.
The preoperative diagnosis of breast cancer in the presented patient with SS was established by radiological evaluation followed by breast biopsy. Lesion morphology, mammary gland density, and the lack of imaging technique skills may limit the sensitivity for detecting breast cancer. As such, mammography may not demonstrate up to 20-30% of malignancies in a dense breast pattern [6,7], as we encountered in the present case.
Although the incidence of axillary metastases in patients with DCIS is small, the evidence of axillary lymph node conglomerates on both physical and radiological examinations in this case led us to a clinical impression of a metastatic or "N2" disease in the TNM staging definition [8], and that is why axillary dissection, in addition to a mastectomy, was performed. But, no axillary metastases were observed on the histopathology results. This clinically positive but pathologically negative node status was then attributed to the SS. We reviewed the literature and did not find any report regarding the management of such patients with SS presenting with breast cancer and an axillary clinicopathological discrepancy. We recommend that lymph node status be verified either pre- or intra-operatively by lymph node biopsy using either fine-needle, core-needle, or frozen-section procedures before establishing a definitive diagnosis and appropriate surgical treatment.
In conclusion, clinicians should remain vigilant to the possibility of overstaged breast cancer due to the false clinical impression of axillary metastasis in patients with SS presenting with breast cancer. Therefore, axillary node status should be verified first to correctly establish tumor stage in this very rare clinical situation.
Figures and Tables
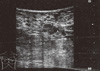 | Figure 1Ultrasonographic appearance of the breast mass. The mass is a hypoechogenic lesion with irregular margins measuring 4 cm in diameter. |
References
2. Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. 2004. 164:1275–1284.

3. Zhang W, Feng S, Yan S, Zhao Y, Li M, Sun J, et al. Incidence of malignancy in primary Sjögren's syndrome in a Chinese cohort. Rheumatology (Oxford). 2010. 49:571–577.

4. Lazarus MN, Robinson D, Mak V, Møller H, Isenberg DA. Incidence of cancer in a cohort of patients with primary Sjögren's syndrome. Rheumatology (Oxford). 2006. 45:1012–1015.

5. Yoshimura M, Koizumi K, Satani K, Kakizaki D, Kawanishi Y, Ohyashiki K, et al. Gallium-67 scintigraphic findings in a patient with breast lymphoma complicated with Sjögren syndrome. Ann Nucl Med. 2000. 14:227–229.

6. Boetes C, Mus RD, Holland R, Barentsz JO, Strijk SP, Wobbes T, et al. Breast tumors: comparative accuracy of MR imaging relative to mammography and US for demonstrating extent. Radiology. 1995. 197:743–747.

7. Ernster VL, Ballard-Barbash R, Barlow WE, Zheng Y, Weaver DL, Cutter G, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002. 94:1546–1554.

8. Clinical practice guidelines in oncology - v.1: breast. National Comprehensive Cancer Network. 2009. Accessed March 8th, 2011.
http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download