INTRODUCTION
Primary breast lymphoma is a rare neoplasm of the breast with an incidence of less than 0.6% of primary breast malignancies. Distinguishing it from breast cancer is important given that their treatments differ radically [1]. Its low incidence is attributed to the relatively small amount of lymphoid tissue in the breast compared to other organs. Primary breast lymphoma is an even rarer neoplasm in the male breast. To the best of our knowledge magnetic resonance imaging (MRI) has not been used previously in the evaluation of breast lymphoma. We report a rare case of male breast lymphoma evaluated with MRI as well as with other conventional imaging modalities, confirmed with ultrasound guided core needle biopsy, and managed by chemo-radiotherapy.
CASE REPORT
A 48-year-old male presented with a unilateral palpable mass in the left breast. None of the risk factors for male breast cancer such as prior irradiation of the chest, exogenous estrogen treatment, liver disease, hyperestrogenism, androgen deficiency or family history had been positive.
On routine mammography, there was a lobulated homogenous well-circumscribed soft tissue mass in the left breast with a large axillary lymph node showing loss of central fatty hilum. No calcifications (micro/macro) could be detected.
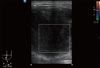
Ultrasound (US) (Philips HD11XE; Philips Ultrasound, Andover, MA, USA; Probe: 12 to 3 Mhz Broadband linear array transducer) revealed a homogenous, lobulated, hypoechoic mass showing mildly increased vascularity (Figures 1, 2).
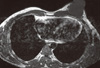
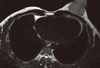
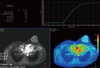
On conventional MRI (Philips MR Systems Achieva; Philips Medical Systems, The Netherlands; 1.5 T MRI machine), the lesion was seen as a well-defined lobulated lesion involving the superomedial and inferomedial quadrants of the left breast, measuring 3.8 × 3.5 × 2.8 cm, appearing isointense to muscle on T1- and mildly hyperintense to muscle on T2-weighted images (Figures 3, 4). The lesion was best delineated on fat suppressed T1-weighted images (T1 SPIR) and showed mild homogenous contrast enhancement and Type II perfusion curve, which indicates initial rapid or slow rise followed by a plateau in the delayed phase (Figure 5) on MRI suggestive of neoplastic etiology.
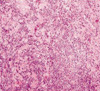
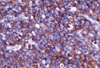
Primary breast lymphoma was the leading diagnosis in this case, based on the combination of clinical and imaging findings. There was no evidence of abdominal or cervical lymphadenopathy. US-guided fine needle aspiration cytology followed by trucut biopsy (Figure 6) confirmed the diagnosis of diffuse large B cell lymphoma which was CD 20 positive on immunohistochemical analysis (Figure 7).
The patient was followed-up for last 7 months and underwent chemotherapy with cyclophosphamide, hydroxydoxorubicin, vincristine and prednisone (CHOP) regimen-14 cycles, resulting in a significant reduction in the size of the mass on physical examination.
DISCUSSION
Primary breast lymphoma is rare with 293 cases reported in the literature [1]. The age of incidence has ranged from 9 to 85 years old, while most frequently seen in the sixth decade. In developed countries, male breast cancer accounts for 1-5.7% of breast cancer cases and 0.2-1.5% of all cases of malignancies in males [2,3]. Forty-four percent of cases of breast lymphoma are primary, although 22% are manifestations of disseminated disease and 29% represent recurrence of preexisting lymphoma [4].
The clinical and histologic criteria for diagnosis of a primary breast lymphoma are as follows [5]: 1) a close association between breast tissue and infiltrating lymphoma; 2) no evidence of widespread lymphoma and no history of previous extramammary lymphoma; and 3) documentation of the breast as the principal organ involved and the primary site.
The differentials considered on mammography were abscess/hematoma and invasive ductal carcinoma. On US, abscess and hematoma could be differentiated from primary breast lymphoma by the lack of vascularity within these two entities [2]. On US, primary breast lymphoma usually presents as a solitary, hypoechoic mass showing increased vascularity with no calcification or necrosis within [6]. Invasive ductal carcinoma, which is much more common than primary breast lymphoma, usually presents with a mass but also demonstrates one or more of the following: calcification, architectural distortion, and spiculated margin. These features are rarely seen in primary breast lymphoma [3]. Metastatic disease to the breast, such as melanoma, lymphoma, sarcoma, lung cancer, and gastric carcinomas, although rare can have a similar appearance to primary breast lymphoma but are usually multiple and bilateral, whereas primary lesions of the breast are single and unilateral. In this case, metastatic disease was a rather low possibility in the differential diagnosis, as this mass was single and unilateral with no known primary malignancy. On MR spectroscopy, malignant breast lesions show prominent choline peak, which is not generally detectable in normal breast tissue [7]. Diffusion MRI has a role in the follow-up of patients with diffuse large B cell lymphoma on chemotherapy to determine tumor response [8].
Conventional and dynamic MRI has been helpful in determining the number of lesions, chest wall involvement, axillary lymph node involvement, and in predicting the nature of the lesions depending on their kinetics. On conventional MR imaging, lymphoid masses and myxoid fibroadenomas demonstrate high signal intensity on T2-weighted image as these are solid, highly cellular masses [9]. It is uncommon for other breast neoplasms to show high signal on T2-weighted images, unless there is intralesional necrosis or a colloid type of ductal carcinoma. On dynamic MRI, there was a rapid early enhancement followed by plateau in the delayed phase suggestive of Type II perfusion curve which is explained by the pattern of enhancement of lymphoma which is usually mild to moderate sustained enhancement.
Primary breast lymphoma can be diagnosed by MRI based on its characteristic imaging features as a single, lobulated, unilateral mass lesion, showing high signal intensity on T2-weighted imaging, mild to moderate homogenous enhancement, Type II perfusion curve, and lack of central necrosis with histological diagnosis further assisting in characterizing the lesion and influencing treatment which includes radiotherapy and chemotherapy. It is important to make an accurate diagnosis, as surgical resection is generally not needed and patients have a better prognosis than in cases of carcinoma of the breast or patients with extranodal lymphomas, where a multidisciplinary approach where combinations of surgery, radiotherapy, and chemotherapy is required [10].




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download