Abstract
Purpose
The frequency of immediate breast reconstruction (IBR) is increasing, and the types of reconstruction used are diverse. Adjuvant chemotherapy is a life-saving intervention in selected high-risk breast cancer patients. The aim of our study was to determine how IBR and type of reconstruction affect the timing of the initiation of chemotherapy.
Methods
We obtained data from female breast cancer patients treated by mastectomy with IBR (IBR group) and without IBR (mastectomy only group) who received adjuvant chemotherapy between January 1, 2008, and December 31, 2010. We retrospectively collected data including patient characteristics, disease characteristics, treatment details, and treatment outcomes from our institutional electronic patient database and medical treatment records. The reconstruction types were categorized as deep inferior epigastric perforator (DIEP) flap, latissimus dorsi (LD) flap and tissue expander/implant (TEI).
Results
In total, 595 patients were included in this study. Of these patients, 43 underwent mastectomy with IBR (IBR group) and 552 patients did not undergo reconstruction (mastectomy only group). There was significant difference in the timing of the initiation of chemotherapy between the two groups (p<0.0001). There were no cases of delays of more than 12 weeks. In the IBR group, 20 patients received TEI, 9 patients were treated by the insertion DIEP flaps, and 14 patients were treated by LD flaps. There were no significant differences in the timing of chemotherapy according to the type of reconstruction (p=0.095).
The frequency of breast reconstruction after mastectomy for breast cancer has increased. Immediate breast reconstruction (IBR) is associated with improved psychosocial recovery, especially when performed at the time of the mastectomy [1-3]. A study has demonstrated the benefits of IBR, which include improved psychological and aesthetic outcomes [4].
Adjuvant chemotherapy is a life-saving intervention in selected high-risk breast cancer patients. According to current guidelines, chemotherapy should be initiated within 4-12 weeks after surgery [5,6]. Previous reports have shown that survival does not differ among patients of whom chemotherapy was initiated within 1, 2, or 3 months after surgery. Delay beyond 3 months was, however, associated with increased disease-specific mortality and overall mortality [7,8]. One barrier to the use of IBR is the concern that the complications of immediate reconstruction may delay wound healing and initiation of adjuvant chemotherapy [9]. Several studies have been carried out of the potential delay of adjuvant treatment after IBR. However, various publications have also demonstrated that IBR is safe and does not delay the start of adjuvant chemotherapy [4,10].
Various types of reconstruction may be performed after mastectomy, but little is known about the impact of the type of IBR on the administration of adjuvant chemotherapy. To determine whether the timing of chemotherapy is affected by immediate reconstruction and the type of IBR, we performed a retrospective study of patients.
We obtained data from female breast cancer patients treated by mastectomy with IBR (IBR group) and without IBR (mastectomy only group) who received adjuvant chemotherapy at the Samsung Medical center between January 1, 2008, and December 31, 2010. We retrospectively collected data including patient characteristics, disease characteristics, treatment details, and treatment outcomes from our institutional electronic patient database and medical treatment records. Details included age at diagnosis, body mass index (BMI), histopathological staging and tumor size, number of positive nodes, date of mastectomy and immediate reconstruction, type of reconstruction, date of removal of drain and discharge, start of adjuvant chemotherapy and wound complications. When the drainage fluid was 30 mL or less in a 24-hour period, it was removed. Patients were staged according to the American Joint Committee on Cancer (AJCC, 7th edition) categories.
All patients underwent total mastectomy (TM) of the affected breast and ipsilateral axillary lymph node dissection (ALND) or sentinel lymph node (SLN) biopsy. In some cases, patients underwent one or more partial mastectomies (PM) before TM, and some had ALND or SLN biopsy at the time of a prior PM. In all patients of the study group, at the time of TM, IBR was performed by a plastic surgeon. The reconstruction types were categorized as deep inferior epigastric perforator (DIEP) flap, latissimus dorsi (LD) flap, and tissue expander/implant (TEI).
The choice of the best flap to use for patients depends on many factors, including body type and breast size, and on the hospital and the plastic surgeon who performs the operation as well as on the patient. In our institution, the plastic surgeons explained to the patients the advantages and disadvantages of each type of reconstruction preoperatively, and the choice was then left entirely to the patient's judgment.
In cases of TEI, expansion is normally performed 2 to 4 weeks after expander insertion and is continued serially 1 to 2 weeks apart until the desired size, at which point overexpansion is performed at up to 20% and continued irrespectively of chemotherapy. The incision should be healing well before subjecting it to expansion. After an additional waiting period of at least 2 months, the expander is then exchanged for a permanent breast implant.
We excluded patients who did not undergo treatment at the Samsung Medical center, who received neoadjuvant (preoperative) chemotherapy, and who delayed their own adjuvant therapy. Patients who participated in other studies associated with adjuvant chemotherapy were excluded from this analysis, since initiation of adjuvant therapy may have been delayed due to assays performed in these studies.
Categorical data were analyzed by chi-square analysis. An independent-sample t-test was used to determine differences in age, BMI, tumor size, number of positive nodes, mean interval to the removal of surgical drains, time of discharge from the hospital and time of initiation of chemotherapy between the IBR group and mastectomy only group. One-way ANOVA was used to compare the characteristics of the three groups with IBR. Probability values of <0.05 were considered significant. Statistical analyses were performed using PASW Statistics 18.0 software (SPSS Inc., Chicago, USA).
Between January 1, 2008 and December 31, 2010, 1,046 female patients with breast cancer underwent mastectomy at our institution, and 683 of these patients received adjuvant chemotherapy. Nine patients who delayed their own adjuvant therapy and 79 patients who had participated in other studies associated with adjuvant chemotherapy were excluded. In total, 595 patients were included in this study. Of these patients, 43 underwent mastectomy with IBR (IBR group) and 552 patients did not undergo reconstruction (mastectomy only group). In the IBR group, 20 patients received TEI, 9 patients were treated by reconstruction with DIEP flaps, and 14 patients were treated by reconstruction with LD flaps.
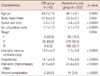
The characteristics of the two groups are summarized in Table 1. The patients in the mastectomy only group were older and more obese than those in the IBR group (p=0.0001 and p=0.002, respectively). The patients in the mastectomy only group were more likely to have larger tumors (p<0.0001), greater numbers of positive nodes (p<0.0001) and more advanced disease (p=0.004) than the patients in the IBR group. There were significant differences in the time intervals prior to the removal of surgical drains, discharge from the hospital and start of chemotherapy between the two groups (p<0.0001, p<0.0001, and p<0.0001, respectively) (Figure 1). There was a statistically significant difference in wound complications between the two groups (p<0.0001) (Table 1).
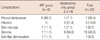
Wound complications include wound dehiscence, infection and skin flap necrosis, seroma, and skin flap demarcation (Figure 2). The most common postoperative complication is seroma (43.5%, 10 of 23) (Table 2). Skin flap necrosis was defined as full-thickness skin loss. Infectionous complications included both those requiring oral antibiotics and treatment in the outpatient setting as well as those requiring hospital admissions for intravenous antibiotic therapy (Table 2). There were significant differences in the time intervals prior to removal of surgical drains, discharge from hospital and start of chemotherapy between the patients with and without wound complications (Table 3).
There were no cases of delay in initiation of chemotherapy of more than 12 weeks after surgery in the two groups. Also, in all patients with advanced (stage III) disease who underwent IBR, chemotherapy was started within 5 weeks of the surgery (range, 20-32; mean, 24.25 days).
We performed a subset analysis to determine if the type of reconstruction influenced the start of chemotherapy. The characteristics of the type of reconstructions are summarized in Table 4. There were no significant differences among the groups for age, BMI, or tumor size. The mean time for the removal of surgical drains for each group was as follows: DIEP flap 8.7 days (range, 6-19 days), LD flap 14.1 days (range, 11-25 days), and TEI 13.2 days (range, 6-30 days). These differences were especially significant between patients receiving DIEP flaps and LD flaps (p=0.023). The mean time of discharge from the hospital and the start of chemotherapy for each group were as follows: DIEP flap 10.8 days (range, 7-20 days) and 27.1 days (range, 21-40 days), respectively, LD flap 14.6 days (range, 11-25 days), and 36.1 days (range, 18-62 days), respectively, and TEI 15.2 days (range, 9-31 days) and 30.2 days (range, 20-52 days), respectively. These differences were not significant (p=0.064, p=0.063). There were differences in the number of positive nodes and wound complications among the groups (p=0.034, p=0.002), but there was no significant difference in the timing of initiation of chemotherapy.
Since IBR enhances psychological recovery from breast cancer [1-3] and seems to be oncologically safe [11,12], IBR procedures have been widely implemented in oncoplastic surgical practice [13-16]. IBR is now established as a valuable option for patients undergoing mastectomy. Early fears that this technique would lead to increased rates of local recurrence or difficulty in detecting and subsequently treating recurrence have been dispelled by several published series [17-19]. The most widely used reconstruction techniques are tissue expanders (later substituted with a permanent implant), or pedicled or free flap procedures [20-22].
A number of studies have been carried out on the effects of IBR on the time interval between surgery and the initiation of adjuvant chemotherapy, as a response to the suggestion that undue delay can compromise cancer treatment and consequently, survival [9,10]. In a study that reviewed the effect of the reconstruction methods (free TRAM and DIEP flaps on the time of commencement of adjuvant treatment), it was demonstrated that there is a delay in the administration of adjuvant treatment in the IBR free flap group compared to patients who did not undergo reconstruction. Patients receiving IBR experienced significant 2-week delays to the initiation of chemotherapy on average compared to the control group [23]. Another study found that in seven of the 28 TRAM IBR patients, their postoperative chemotherapy was delayed due to surgical complications [24]. In a study that reviewed the impact of breast reconstruction on the delivery of chemotherapy, IBR does not appear to lead to the omission of chemotherapy, but it is associated with a modest, but statistically significant, delay in initiating treatment [25]. In contrast, a study demonstrated that IBR did not lead to a delay in the delivery of chemotherapy when compared to conservation surgery or non-reconstructive mastectomy [6].
In our study, the patients in the IBR group were younger than the patients in the mastectomy only group (p=0.0001). Other studies have recognized the same trend [26-28]. This likely represents patient preference, with younger patients more actively seeking reconstruction, as well as physician bias, with physicians encouraging younger patients (and less actively encouraging older patients) to have immediate reconstruction. Patients undergoing mastectomy alone were more likely to have large tumors (p<0.0001), several positive nodes (p<0.0001) and more advanced stages of the disease (p=0.004). This probably reflects the fact that patients with advanced disease are required to be candidates for adjuvant radiation therapy, and therefore IBR is often not considered. Recommendations regarding IBR may be influenced by the likelihood that the patient will also require adjuvant postmastectomy chest wall and nodal radiation. Reconstruction may affect the technical delivery of radiation, and radiation may adversely affect the cosmetic results of reconstruction [29,30]. This concern leads some oncologists to recommend delayed reconstruction for women who are likely to receive radiation. The patients in the mastectomy only group were more obese than those in the IBR group (p=0.002), but this difference was not significantly correlated with a delay in the initiation of chemotherapy.
There were significant differences in the time intervals for the removal of surgical drains, the discharge from the hospital and the start of chemotherapy, and in the wound complications between the two groups (p<0.0001 for all factors). There was a correlation between the delay in initiation of chemotherapy and the time for the removal of surgical drains, and discharge from the hospital. This study examined the effects of reconstruction on the timing of the administration of chemotherapy. Although IBR was associated with an increased delay in chemotherapy initiation compared with the mastectomy only group, IBR did not increase the chance that adjuvant chemotherapy would be omitted. Some studies have found no differences in survival between patients given chemotherapy early, compared with those who received treatment up to 12 weeks postoperatively [7,8]. However, delays over 3 months are associated with diminished relapse-free survival and overall survival [7]. In our series, all of the breast cancer patients who received chemotherapy started treatment within 12 weeks postoperatively. In all patients with advanced (stage III) disease who underwent IBR, chemotherapy was started within 5 weeks of the surgery.
Another aim of this study was to determine whether the type of reconstruction influenced the start of chemotherapy. In one study, half of the patients who underwent IBR with free tissue transfer had their adjuvant therapy delayed by more than 40 days. Although there were no significant differences among the IBR types and mastectomy alone patients, the free tissue transfer subgroup did seem to have a statistically significant increase of 36% in the delay [6]. In this study, subgroup analysis based on type of reconstruction was somewhat limited due to the small numbers of patients in the subgroups, but no significant differences were found in age, BMI, or tumor size between the various types of reconstruction (Table 4). Meanwhile, although there was a significant difference in the time of the removal of surgical drains between the DIEP flap group and the LD flap group, the mean times of discharge from the hospital and start of chemotherapy for each group did not significantly differ. There were significant differences in the number of positive nodes and wound complications among the groups (p=0.034, p=0.002). The rates of wound complication were highest in the LD flap group and lowest in the TEI group. Although the mean time for the start of chemotherapy did not significantly differ, it was longer in the LD flap group than in the other groups.
Finally, in this study, IBR was not associated with a clinically significant delay or omission of adjuvant chemotherapy. However, it was associated with a statistically significant delay in initiating treatment. Therefore, for patients who are at higher risk, the additional delay associated with IBR should be considered. Our data also shows that the type of reconstruction does not affect the delay of the initiation of chemotherapy.
This study is limited by its retrospective design and small number of single-center patients in each group, especially the study group. We also were not able to analyze all types of reconstruction.
There is a significant difference in the timing of initiating adjuvant chemotherapy after mastectomy and IBR in comparison to mastectomy alone. However, IBR does not appear to lead to omission of chemotherapy and the difference was not clinically significant. Further, the type of reconstruction used does not affect the timing of chemotherapy.
Figures and Tables
 | Figure 1Comparison of interval to drain removal and chemotherapy between immediate breast reconstruction (IBR) group and mastectomy only group. There were significant differences in time intervals prior to removal of surgical drains, start of chemotherapy between the two groups (p<0.0001, p<0.0001). (A) Interval to drain removal and frequency (IBR group). (B) Interval to drain removal and frequency (mastectomy only group). (C) Interval to chemotherapy and frequency (IBR group). (D) Interval to chemotherapy and frequency (mastectomy only group). SD=standard deviation. |
 | Figure 2Postoperative wound complication after mastectomy with immediate breast reconstruction. Wound demarcation occurred after latissimus dorsi flap reconstruction. On 42 days after operation, escharectomy and bedside debridement underwent. |
References
1. Dean C, Chetty U, Forrest AP. Effects of immediate breast reconstruction on psychosocial morbidity after mastectomy. Lancet. 1983. 1:459–462.

2. Rosenqvist S, Sandelin K, Wickman M. Patients' psychological and cosmetic experience after immediate breast reconstruction. Eur J Surg Oncol. 1996. 22:262–266.
3. Al-Ghazal SK, Sully L, Fallowfield L, Blamey RW. The psychological impact of immediate rather than delayed breast reconstruction. Eur J Surg Oncol. 2000. 26:17–19.

4. Kim S, Lee S, Lee H, Lee J. The safety and cosmetic effect of immediate latissimus dorsi flap reconstruction after breast conserving surgery. J Breast Cancer. 2009. 12:186–192.

5. Mortenson MM, Schneider PD, Khatri VP, Stevenson TR, Whetzel TP, Sommerhaug EJ, et al. Immediate breast reconstruction after mastectomy increases wound complications: however, initiation of adjuvant chemotherapy is not delayed. Arch Surg. 2004. 139:988–991.

6. Wilson CR, Brown IM, Weiller-Mithoff E, George WD, Doughty JC. Immediate breast reconstruction does not lead to a delay in the delivery of adjuvant chemotherapy. Eur J Surg Oncol. 2004. 30:624–627.

7. Lohrisch C, Paltiel C, Gelmon K, Speers C, Taylor S, Barnett J, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2006. 24:4888–4894.

8. Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat. 2006. 99:313–321.

10. Patani N, Devalia H, Anderson A, Mokbel K. Oncological safety and patient satisfaction with skin-sparing mastectomy and immediate breast reconstruction. Surg Oncol. 2008. 17:97–105.

11. Zakhireh J, Fowble B, Esserman LJ. Application of screening principles to the reconstructed breast. J Clin Oncol. 2010. 28:173–180.

12. Howard MA, Polo K, Pusic AL, Cordeiro PG, Hidalgo DA, Mehrara B, et al. Breast cancer local recurrence after mastectomy and TRAM flap reconstruction: incidence and treatment options. Plast Reconstr Surg. 2006. 117:1381–1386.

13. Pennington DG. Breast reconstruction after mastectomy: current state of the art. ANZ J Surg. 2005. 75:454–458.

14. Heneghan HM, Prichard RS, Devaney A, Sweeney KJ, Malone C, McLaughlin R, et al. Evolution of breast cancer management in Ireland: a decade of change. BMC Surg. 2009. 9:15.

15. Malycha PL, Gough IR, Margaritoni M, Deo SV, Sandelin K, Buccimazza I, et al. Oncoplastic breast surgery: a global perspective on practice, availability, and training. World J Surg. 2008. 32:2570–2577.

16. Association of Breast Surgery at BASO 2009. Surgical guidelines for the management of breast cancer. Eur J Surg Oncol. 2009. 35:Suppl 1. 1–22.
17. Medina-Franco H, Vasconez LO, Fix RJ, Heslin MJ, Beenken SW, Bland KI, et al. Factors associated with local recurrence after skin-sparing mastectomy and immediate breast reconstruction for invasive breast cancer. Ann Surg. 2002. 235:814–819.

18. Simmons RM, Fish SK, Gayle L, La Trenta GS, Swistel A, Christos P, et al. Local and distant recurrence rates in skin-sparing mastectomies compared with non-skin-sparing mastectomies. Ann Surg Oncol. 1999. 6:676–681.

19. Newman LA, Kuerer HM, Hunt KK, Kroll SS, Ames FC, Ross MI, et al. Presentation, treatment, and outcome of local recurrence afterskin-sparing mastectomy and immediate breast reconstruction. Ann Surg Oncol. 1998. 5:620–626.

20. Elliott LF, Eskenazi L, Beegle PH Jr, Podres PE, Drazan L. Immediate TRAM flap breast reconstruction: 128 consecutive cases. Plast Reconstr Surg. 1993. 92:217–227.
23. Kontos M, Lewis RS, Lüchtenborg M, Holmberg L, Hamed H. Does immediate breast reconstruction using free flaps lead to delay in the administration of adjuvant chemotherapy for breast cancer? Eur J Surg Oncol. 2010. 36:745–749.

24. Schusterman MA, Kroll SS, Weldon ME. Immediate breast reconstruction: why the free TRAM over the conventional TRAM flap? Plast Reconstr Surg. 1992. 90:255–261.

25. Alderman AK, Collins ED, Schott A, Hughes ME, Ottesen RA, Theriault RL, et al. The impact of breast reconstruction on the delivery of chemotherapy. Cancer. 2010. 116:1791–1800.

26. Morrow M, Scott SK, Menck HR, Mustoe TA, Winchester DP. Factors influencing the use of breast reconstruction postmastectomy: a National Cancer Database study. J Am Coll Surg. 2001. 192:1–8.

27. Recht A, Come SE, Henderson IC, Gelman RS, Silver B, Hayes DF, et al. The sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer. N Engl J Med. 1996. 334:1356–1361.

28. Pusic A, Thompson TA, Kerrigan CL, Sargeant R, Slezak S, Chang BW, et al. Surgical options for the early-stage breast cancer: factors associated with patient choice and postoperative quality of life. Plast Reconstr Surg. 1999. 104:1325–1333.

29. Spear SL, Ducic I, Low M, Cuoco F. The effect of radiation on pedicled TRAM flap breast reconstruction: outcomes and implications. Plast Reconstr Surg. 2005. 115:84–95.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download