Abstract
Purpose
The aims of this study were to investigate outcomes corresponding to age at diagnosis as categorized into 5-year intervals and to explore whether endocrine-responsive tumors display clinical benefits from endocrine therapy after chemotherapy among young breast cancer patients.
Methods
A total of 1,171 patients who were under 40 years old at diagnosis between 1985 and 2007 were divided into 3 subgroups: ≤30 years (Group I, 13.3%), 31-35 years (Group II, 30.5%), and 36-40 years (Control group, 56.2%). Clinicopathological factors and outcomes were compared using a chi-square test, the Kaplan-Meier method, and Cox's hazards models.
Results
There were no significant differences in the characteristics and treatment patterns between the 3 groups, except for the grade, hormone receptors expression, and use of endocrine therap. Group I showed the worst survival and subsequently Group II presented worse outcomes than the Control group, mainly among hormone receptors-positive patients. Groups I and II showed increased risks of recurrence and death in multivariate analyses. Among 529 hormone receptors-positive patients who received chemotherapy, favorable outcomes for patients who were treated with endocrine agents were demonstrated, mainly in patients aged 35 years or less. However, interaction tests between the use of endocrine therapy and age at diagnosis were not significant.
Conclusion
Age at diagnosis is an independent prognostic factor and the age of 35 years is a rational cut-off among young patients. Our subgroup analysis suggests that endocrine therapy may provide additional benefits even in young breast cancers. Therefore, further researches should be directed towards improving outcomes for this population.
Worldwide, breast cancer is the most common cancer in women and its incidence has been increasing every year, reaching up to 22.9% of all female malignancies [1,2]. Likewise, breast cancer in Korea remains the second most common cancer following thyroid cancer [3]. The peak age of breast cancer is in the 60s in Western countries and women under 35 years old at the time of diagnosis accounts for less than 4% of all breast cancer patients [4]. However, in Korea, the peak age is in the 40s and the proportion of patients of this age is much higher than it is in the West [4,5]. Although it has been reported that younger patients generally have poorer prognoses than older patients, there are still debates on what the optimal cut-off age for defining a young age is, or whether the age itself serves as a prognostic factor. A recent study using the large nationwide Korean Breast Cancer Society (KBCS) registry suggests that an age of 35 years at the time of diagnosis is a reasonable cut-off point for the determination of a young age for breast cancer [6,7]; however, disease recurrence is not investigated according to age at diagnosis [6].
Patients aged less than 35 years at diagnosis have been considered as an important risk category [8]. Therefore, these young patients often require systemic chemotherapy. However, younger patients treated with chemotherapy alone showed an greater risk of relapse and death than older patients if their tumors expressed estrogen receptors (ER) [9,10]. Although a retrospective study suggests that chemotherapy-induced amenorrhea (CIA) is likely to provide clinical benefits to premenopausal hormone receptors-positive breast cancer patients [11], the endocrine effects of chemotherapy alone are insufficient for young patients with ER-positive tumors. The Early Breast Cancer Trialists' Collaborative Group overview found that treatment with adjuvant tamoxifen for 5 years significantly reduces disease recurrence and death in ER-positive women under 50 years old, irrespective of chemotherapy treatment [12]. However, there was no subgroup analysis for the specialized case of young women under the ages of 35 or 40 years. Furthermore, a recent study demonstrated no significant additional survival benefit resulting from endocrine therapy in hormone receptor-positive patients less than 35 years old who received chemotherapy [13]; however, a limited number of patients younger than 35 years with hormone receptor-positive tumors (n=314) were analyzed due to many missing values.
The aims of this study were to investigate disease recurrence and survival according to age at diagnosis (as categorize into 5-year intervals) among young breast cancer patients aged 40 years or less and to explore whether young patients with endocrine-responsive tumors obtain additional clinical benefits from adjuvant endocrine therapy after administration of chemotherapy.
Patients were selected from the Severance Hospital Breast Cancer Registry Database, which contains clinicopathological information, treatment modalities, and details of outcomes. Between January 1985 and December 2007, 5,582 patients with breast cancer were treated at the Severance Hospital, Yonsei University Health System, in Seoul, Korea. The exclusion criteria were as follows: pure in situ carcinoma of the breast (n=506), recurrent or metastatic disease (n=50), non-epithelial origin tumor such as phyllodes tumor, lymphoma, or sarcoma (n=52), incomplete data for tumor and nodal status (n=21), and age at diagnosis over 40 years (n=3,782). A total of 1,171 invasive breast cancer patients, whose ages at diagnosis were 40 years or less, were finally enrolled for analyses. The patients were divided into 3 groups according to their age at diagnosis: Group I, aged 30 years or less; Group II, aged between 31 and 35 years; and the Control group, aged between 36 and 40 years. We compared clinicopathological characteristics, treatment patterns, and survival outcomes among the groups.
Patients were treated with either total mastectomy or breast-conserving surgery and sentinel lymph node biopsy or axillary lymph node dissection. After surgery, local radiotherapy or adjuvant systemic treatments were administered if the patient was able to tolerate it. Patients with hormone receptors-positive or -unknown (n=28) tumors received daily endocrine therapy with 20 mg of tamoxifen for 5 years. Forty patients received subcutaneously 3.6 mg of goserelin acetate every 4 weeks for 2 years. The clinical follow-up included history taking, physical examination, laboratory tests, and radiological imaging tests every 6-12 months for detection of relapse. The classification of the tumor stage was based on the American Joint Committee on Cancer 6th edition criteria. The histological grade was assessed by the modified Bloom-Richardson classification. Tumors with ≥10% nuclear-stained cells were considered to have ER and progesterone receptor (PR) positivity. HER2/neu immunohistochemical staining was scored from 0 to 3+ according to the guidelines indicated for HercepTest™ (Dako, Glostrup, Denmark) [14]. Because the fluorescence in situ hybridization (FISH) test had not been performed routinely during most of the study period, HER2 staining was considered positive when strong (3+) membranous staining was observed, whereas cases with 0 to 2+ were regarded as negative.
Locoregional recurrence was defined as tumor recurrence in the ipsilateral breast, chest wall, and regional lymph node. Any recurrence at a distant site including the contralateral axillary or supraclavicular lymph nodes was considered as a distant metastasis. Disease-free survival (DFS) was measured from the date of the first curative surgery to the date of the first locoregional or systemic recurrence or death before any type of relapse. Locoregional relapse-free survival (LRRFS) was calculated from the date of the first operation to the date of the first locoregional relapse or death without any type of recurrence. Distant relapse-free survival (DRFS) was measured from the date of the first operation to the date of the first distant metastasis or death without any type of recurrence. Overall survival (OS) was calculated from the date of the first surgery to the date of the last follow-up or death from any cause.
Comparisons between categorical variables were assessed using Pearson's chi-square test or Fisher's exact test. Survival curves were plotted using the Kaplan-Meier method. Intergroup differences in the survival time were accessed by the log-rank test. Multivariate analyses were carried out using the Cox proportional hazards model. All statistical tests were two-sided and a p-value <0.05 was considered statistically significant. The PASW® Statistics 18 (SPSS Inc., Chicago, USA) was used for all statistical analyses.
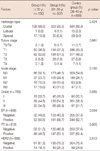
Among a total of 1,171 patients eligible for this study, 156 (13.3%) were categorized into Group I, 357 (30.5%) were Group II, and 658 (56.2%) were a Control group of premenopausal breast cancer patients aged between 36 and 40 years. The mean age at diagnosis and the follow-up duration were 35.3 years old and 72.2 months, respectively. The characteristics of each subgroup are demonstrated in Table 1. Most of our study population showed ductal type (n=1,045, 89.2%) carcinomas and 106 (9.1%) showed special types, including papillary, mucinous, medullary, tubular, metaplastic, and apocrine carcinomas. There were no significant differences in histologic type, tumor and node stage among the 3 groups. Among patients who were available for histologic grading (n=780, 66.6%), Group I patients showed frequent poorly-differentiated tumors. Group I or II patients presented a higher proportion of ER- or PR-negative tumors. HER2 expression was not different among the 3 groups.
Details of treatment patterns are presented in Table 2. Among our study population, 116 (9.9%) patients with locally advanced breast cancer received preoperative chemotherapy containing an anthracycline-based regimen with or without taxane. Group I patients more frequently received neoadjuvant chemotherapy based on taxane regimens, but no significant difference was found for regimens of neoadjuvant chemotherapy (data not shown). Local therapeutic modalities were also not different among the 3 groups. Of 1,161 patients, 982 (84.6%) received adjuvant chemotherapy and no significant difference in the use of chemotherapy was determined among the 3 groups. Endocrine therapy was more frequently administered in the Control group, because endocrine-responsive tumors were significantly higher in those patients (Table 1).
Five-year DFS, LRRFS, DRFS, and OS rates of our whole study population were 73.5%, 78.2%, 76.4%, and 83.6%, respectively. The Kaplan-Meier survival curves according to age at diagnosis are shown in Figure 1. Group I patients showed the worst survival and subsequently Group II presented worse outcomes than the Control group (Figure 1A for DFS, p=0.014; Figure 1B for LRRFS, p=0.001; Figure 1C for DRFS, p=0.009; and Figure 1D for OS, p<0.001, respectively). There was no statistically significant difference in the survival outcomes between Group I and Group II patients, although Group I showed poorer survival than Group II. These significant associations were statistically maintained in 625 hormone receptors-positive patients (DFS, p=0.003; LRRFS, p<0.001; DRFS, p=0.004; and OS, p<0.001, respectively), but not in 293 patients of the hormone receptors-negative subgroups (DFS, p=0.313; LRRFS, p=0.305; DRFS, p=0.209; and OS, p=0.081, respectively). In multivariate analyses, being in Group I or Group II was revealed to be significant predictor of poorer disease outcomes and survival after adjustment for tumor and nodal status, ER, and treatment with chemotherapy and endocrine therapy (Table 3). Also, as we expected, the tumor stage, nodal status, and use of systemic chemotherapy were significant prognostic factors among our young breast cancer patients.
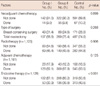
To explore whether the use of endocrine therapy provides additional clinical benefits to hormone receptors-positive patients treated with systemic chemotherapy, clinicopathological characteristics of 529 patients with ER- and/or PR-positive tumors who received chemotherapy were investigated according to treatment with endocrine therapy (Table 4). In patients with smaller tumor sizes and ER-positive or PR-positive tumors, endocrine therapy was more frequently administered. Survival curves of endocrine-responsive breast cancer patients treated with chemotherapy are shown in Figure 2 according to the use of endocrine therapy among all subgroups (n=529; Figure 2A, B), patients aged 35 years or less (n=201; Figure 2C, D), and patients aged between 36 and 40 years (n=328; Figure 2E, F). The use of endocrine therapy provided additional survival benefits to all of the hormone receptors-positive subgroups that received systemic chemotherapy. The statistical significance was especially maintained in patients aged 35 years or less, but not in those between 36 and 40 years. When tumor and node stage were both entered into the Cox model, however, formal interaction tests between the use of endocrine therapy and age at diagnosis (age of 35 years or less versus age between 36 and 40 years) were not significant for DFS (hazard ratio [HR], 1.375; 95% confidence interval [CI], 0.712-2.656; p=0.343) and OS (HR, 1.105; 95% CI, 0.490-2.493; p=0.810); therefore, statistical interpretation should be done with caution.
Through this study, we found that patients aged 30 years or less show the highest risk of disease recurrence and death, and those between 31 and 35 years also display significantly poorer outcomes compared to control groups after adjusting for important clinicopathological parameters among young breast cancer patients. These results are consistent with a recent study by the KBCS [6]. According to previous retrospective studies, younger patients had worse prognoses than older patients [4, 15]. Although the control groups of those studies included many premenopausal patients in their 40s, our unique study population was composed of relatively younger breast cancer patients. Although our study with its relatively small sample size did not demonstrate significant differences in disease relapse and survival between Group I and Group II, our study again supported that an age of 35 years at the time of diagnosis is a reasonable cut-off value for defining young age breast cancers [6,7].
Recent studies have shown that the disease recurrence rate is higher in young breast cancer patients [16,17]. Our earlier report demonstrated that patients aged less than 35 years showed poorer LRRFS and DRFS [18]. These results were caused not by associated pathological features but by different tumor biology based on patient age at diagnosis and being under the age of 35 years at the time of diagnosis was an independent prognostic factor in breast cancer patients. Although there were some missing values, grade III, ER-negative, and PR-negative tumors, often showing highly proliferative features, were significantly associated with patients under 35 years and when all of these parameters were entered into multivariate models in combination with the tumor and node stage and the use of adjuvant therapy, being under the age of 35 years at diagnosis was an independent prognostic factor for disease outcomes (HR, 1.534; 95% CI, 1.156-2.035; p=0.003 for DFS; HR, 1.723; 95% CI, 1.261-2.354; p=0.001 for LRRFS; HR, 1.549; 95% CI, 1.147-2.091; p=0.004 for DRFS; HR, 2.012; 95% CI, 1.406-2.878; p<0.001 for OS). Until now, a limited number of studies have been designed to determine the molecular or genetic differences that occur in younger breast cancer patients. A recent study using comparative genomic hybridization analyses suggested that specific chromosomal alterations might result in a poor prognosis for early-onset breast cancer [19]. Further studies should be attentive to molecular levels to confirm the biology of early-onset breast cancer.
Kroman and his colleagues [20] reported that age did not have a significant effect on the survival of women who received adjuvant cytotoxic treatment, but that it was highly implicated among low risk patients who did not receive adjuvant chemotherapy. In an earlier report, Fisher et al. [21] showed that adjuvant chemotherapy is beneficial for patients with low risk factors and is most advantageous in premenopausal women. These researches may imply that for young breast cancer patients, even in the early stages of cancer, more aggressive treatments may be necessary. However, BRCA1 and BRCA2 mutations are found more frequently in young women with breast cancer and are often associated with bilateral tumors or triple negative breast cancers, which have limited therapeutic options and show poorer outcomes [22-24]. Intensive surveillance and newly tailored therapies such as poly (ADP-ribose) polymerase (PARP) inhibitors should be considered [25,26].
The difference in prognosis according to age at diagnosis might be associated with hormonal mechanisms. This finding is consistently demonstrated only in hormone receptors-positive tumors [6,7,13]. However, it is controversial as to whether the additional use of tamoxifen after the administration of chemotherapy provides significant clinical benefits to younger patients. In 2007, Ahn et al. [13] demonstrated no significant survival benefit from endocrine therapy in patients younger than 35 years with hormone receptors-unknown or -positive tumors and suggested that the poor survival of very young patients with hormone receptors-positive tumors was associated with tamoxifen resistance. This result was different from that of our subgroup analysis. Although crosstalk between ER and HER2 signals might be related to this result, HER2 overexpression was not significantly different regardless of age at diagnosis (Table 1) or use of endocrine therapy (Table 4), in agreement with a previous report by the KBCS [13]. Many missing HER2 results and no FISH test in the present study means that our subgroup analysis requires further investigation using an independent dataset.
Although interaction tests did not reveal statistical significance, favorable survival was demonstrated in young hormone receptors-positive patients, especially those aged 35 years or less, who received both chemotherapy and endocrine therapy. It might be compounded by regimens of chemotherapy, CIA, adherence to endocrine therapy, or additional use of ovarian function suppression. Although it has not been clearly established whether CIA is beneficial for the survival of premenopausal breast cancer patients, younger women are less likely to experience CIA [11]. The incidence of CIA is known to be associated with regimen, schedule, and dosage of chemotherapy and additional use of tamoxifen in addition to chemotherapy, as well as patient age [11,27]. Approximately half of our Group I and II patients received CMF (cyclophoaphamide, methotrexate, fluorouracil) chemotherapy which was mainly administered before the early 2000s. It is also possible that more young women ceased 5-year tamoxifen therapy than older women due to menopausal symptoms, sexual dysfunction, or desire for pregnancy. Although the effects of additional ovarian function suppression with or without tamoxifen have yet to be determined in premenopausal women who received chemotherapy, a small number of our patients with high risk factors received additional ovarian suppression because of the absence of CIA or recovery of menstruation after chemotherapy. There are many issues to be investigated regarding the impacts of endocrine therapy on the survival of young breast cancer patients and we have to await the results of an ongoing randomized clinical trial (NCT00912548, KBCSG005, ASTRRA).
In conclusion, patients aged 35 years or less show significantly worse prognosis; therefore, an age of 35 years at the time of diagnosis is an independent prognostic factor and a rational cut-off value among young breast cancer patients. These associations are mainly demonstrated in endocrine-responsive tumors: however, the implications of endocrine therapy are not clearly established in young premenopausal patients after the administration of chemotherapy. Different age distributions in Korea to those in Western countries and poorer prognoses for young breast cancer patients create the need for special consideration of this population for improving outcomes. Future studies based on the molecular and genetic levels and prospective clinical trials should be directed towards developing effective, tailored therapies for each patient of this population.
Figures and Tables
 | Figure 1(A) Disease-free, (B) locoregional relapse-free, (C) distant relapse-free, and (D) overall survival curve. The blue line represents Group I, the red line represents Group II, and the green line represents the Control group. |
 | Figure 2(A, C, E) Disease-free and (B, D, F) overall survival of patients treated with chemotherapy according to treatment of endocrine therapy; (A, B) among all subgroups with hormone receptors-positive tumors; (C, D) patients aged 35 years or less; (E, F) patients aged between 36 and 40 years. The blue line represents patients who received both chemotherapy and endocrine therapy and the red line represents those who received only chemotherapy without endocrine therapy. Of 201 patients aged 35 years or less, 153 (76.1%) received chemo-endocrine therapy and 48 (23.9%) received chemotherapy alone. Of 328 patients aged between 36 and 40 years, 264 (80.5%) received chemo-endocrine therapy and 64 (19.5%) received chemotherapy alone. |
ACKNOWLEDGEMENTS
This work was supported by the Brain Korea 21 Project for Medical Science, Yonsei University, and in part by a grant-in-aid from Novartis Korea Co., Astra Zeneca Korea Co., Sanofi-Aventis Pharmaceutical Co. and Dong-A Pharmaceutical Co.
References
1. Elkum N, Dermime S, Ajarim D, Al-Zahrani A, Alsayed A, Tulbah A, et al. Being 40 or younger is an independent risk factor for relapse in operable breast cancer patients: the Saudi Arabia experience. BMC Cancer. 2007. 7:222.

2. Tarone RE. Breast cancer trends among young women in the United States. Epidemiology. 2006. 17:588–590.

3. Shin HR, Jung KW, Won YJ, Park JG. 139 KCCR-affiliated Hospitals. 2002 annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004. 36:103–114.

4. Han W, Kim SW, Park IA, Kang D, Kim SW, Youn YK, et al. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004. 4:82.

5. Ko SS. Korean Breast Cancer Society. Chronological changing patterns of clinical characteristics of Korean breast cancer patients during 10 years (1996-2006) using nationwide breast cancer registration on-line program: biannual update. J Surg Oncol. 2008. 98:318–323.

6. Han W, Kang SY. Korean Breast Cancer Society. Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat. 2010. 119:193–200.

7. Kim EK, Noh WC, Han W, Noh DY. Prognostic significance of young age (<35 years) by subtype based on ER, PR, and HER2 status in breast cancer: a nationwide registry-based study. World J Surg. 2011. 35:1244–1253.

8. Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thürlimann B, Senn HJ, et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005. 16:1569–1583.

9. Aebi S, Gelber S, Castiglione-Gertsch M, Gelber RD, Collins J, Thürlimann B, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000. 355:1869–1874.

10. Goldhirsch A, Gelber RD, Yothers G, Gray RJ, Green S, Bryant J, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001. (30):44–51.

11. Jung M, Shin HJ, Rha SY, Jeung HC, Hong S, Moon YW, et al. The clinical outcome of chemotherapy-induced amenorrhea in premenopausal young patients with breast cancer with long-term follow-up. Ann Surg Oncol. 2010. 17:3259–3268.

12. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005. 365:1687–1717.
13. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea--a report from the Korean Breast Cancer Society. J Clin Oncol. 2007. 25:2360–2368.

14. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007. 25:118–145.

15. Kang BM, Jung JH, Lim YS, Park HY, Lee YH. Clinical significance of age for premenopausal women with primary breast cancer. J Breast Cancer. 2006. 9:323–329.

16. Xiong Q, Valero V, Kau V, Kau SW, Taylor S, Smith TL, et al. Female patients with breast carcinoma age 30 years and younger have a poor prognosis: the M.D. Anderson Cancer Center experience. Cancer. 2001. 92:2523–2528.

17. Albain KS, Allred DC, Clark GM. Breast cancer outcome and predictors of outcome: are there age differentials? J Natl Cancer Inst Monogr. 1994. (16):35–42.
18. Park BW, Kim SI, Kim EK, Yang WI, Lee KS. Impact of patient age on the outcome of primary breast carcinoma. J Surg Oncol. 2002. 80:12–18.

19. Weber-Mangal S, Sinn HP, Popp S, Klaes R, Emig R, Bentz M, et al. Breast cancer in young women (<or=35 years): genomic aberrations detected by comparative genomic hybridization. Int J Cancer. 2003. 107:583–592.

20. Kroman N, Jensen MB, Wohlfahrt J, Mouridsen HT, Andersen PK, Melbye M. Factors influencing the effect of age on prognosis in breast cancer: population based study. BMJ. 2000. 320:474–478.
21. Fisher B, Dignam J, Wolmark N, DeCillis A, Emir B, Wickerham DL, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997. 89:1673–1682.

22. Lee EH, Park SK, Park B, Kim SW, Lee MH, Ahn SH, et al. Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast Cancer Res Treat. 2010. 122:11–25.

23. Son BH, Ahn SH, Lee MH, Park SK, Kim SW. Korean Breast Cancer Society. Hereditary breast cancer in Korea: a review of the literature. J Breast Cancer. 2008. 11:1–9.

24. Han SA, Park SK, Ahn SH, Son BH, Lee MH, Choi DH, et al. The breast and ovarian cancer risks in Korea due to inherited mutations in BRCA1 and BRCA2: a preliminary report. J Breast Cancer. 2009. 12:92–99.

25. Koo DH, Chung IY, Kang E, Han SA, Kim SW. Usage patterns of surveillance, chemoprevention and risk-reducing surgery in Korean BRCA mutation carriers: 5 years of experience at a single institution. J Breast Cancer. 2011. 14:Suppl 1. S17–S23.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download