Abstract
Purpose
Axillary lymph node status is the strongest prognostic indicator of survival for women with breast cancer. The purpose of this study was to evaluate whether sentinel lymph node biopsy (SLNB) is required in patients with an initial diagnosis of ductal carcinoma in situ (DCIS).
Methods
A retrospective analysis was performed of 78 patients with an initial diagnosis of DCIS between December 2002 and April 2010 and who proceeded to have either SLNB or axillary node dissection performed as part of their primary surgical procedure. The study focused on the rates of axillary node metastasis and the underestimation of invasive carcinoma at an initial diagnosis.
Results
Forty-eight patients underwent SLNB and 18 patients underwent axillary node dissection. Only 1 of 66 patients (1.5%) had a positive sentinel lymph node. After definite surgery, the final diagnosis was changed to invasive ductal carcinoma (IDC) in 12 patients and DCIS with microinvasion in 2 patients; 14 of 78 patients (17.9%) were therefore underestimated at preoperative histological examinations. In 35 patients who were diagnosed DCIS by core needle biopsy (CNB), 13 patients (37.1%) were upstaged into IDC or DCIS with microinvasion in the final diagnosis. The statistically significant factors predictive of invasive breast cancer were a large tumor size and HER2 overexpression.
Conclusion
The rates of SLNB positivity in pure DCIS are very low, and there is continuing uncertainty about its clinical importance. However in view of the high rate of underestimation of invasive carcinoma in patients with an initial diagnosis of DCIS, SLNB appears to be appropriate in these patients, especially in the case when DCIS is diagnosed by a core needle biopsy. In patients with an initial diagnosis of DCIS by CNB, SLNB should be considered as part of the primary surgical procedure, when preoperative variables show a tumor larger than 2.35 cm and with HER2 overexpression.
Ductal carcinoma in situ (DCIS) is a preinvasive lesion that has increased in frequency of diagnosis with the extensive use of mammography for screening The rate of lymph node metastasis in pure DCIS is extremely low (≤1%) [1-3] and the need for axillary lymph node dissection (ALND) for DCIS is generally believed to be unwarranted, although axillary lymph node status is the most important prognostic indicator in breast cancer.
Sentinel node biopsy is recommended for patients with invasive breast cancer, although the role of sentinel node biopsy in DCIS is controversial [4,5]. The rates of positive sentinel node biopsy in patients with pure DCIS vary between 2% and 13% [1-3,6,7], and many studies suggest that sentinel node biopsy in pure DCIS can be safely avoided [8-10]. However, other studies reported that high-risk DCIS and DCIS with microinvasion (DCISM) are associated with a high incidence of lymph node micrometatasis [3,11-13].
Furthermore, most preoperative diagnoses of DCIS are diagnosed by core needle biopsy (CNB) which has a higher risk of invasive breast cancer on final pathologic diagnosis, and the reported rate of underestimation varies between 8.3% and 43.6% [14-17].
In this study, we evaluated whether sentinel node biopsy is required in patients with an initial diagnosis of DCIS and we focused on the rates of axillary node metastasis and the underestimation of invasive carcinoma at an initial diagnosis.
A retrospective analysis was performed of 81 patients with an initial diagnosis of DCIS or DCISM at Daegu Catholic University Medical Center, who were reviewed from December 2002 to April 2010.
The patients were diagnosed with DCIS preoperatively by either CNB or excision, except for one patient who was diagnosed by fine needle aspiration (FNA). CNB and FNA were performed under ultrasonography (USG) guidance in all cases. The patients preoperatively underwent mammography, breast USG and FNAC of suspicious axillary lymph nodes. All patients underwent breast surgery such as breast conserving surgery or mastectomy, and sentinel lymph node biopsy (SLNB) or ALND was performed as part of their primary surgical procedure.
All surgical specimens and sentinel lymph nodes were examined histologically with hematoxylin and eosin (H&E) stain. If no metastasis was detected in the sentinel nodes (SNs) on H&E staining, they were evaluated using immunohistochemical (IHC) stain with cytokeratin (CAM 5.2; BD Biosciences, San Jose, USA). SNs were classified as either positive if they contained either macrometastases or micrometastases, or negative if only isolated tumor cells were present. Malignant cells in regional lymph nodes detected by H&E or IHC that were no greater than 0.2 mm were defined as pN0(i+), and no regional lymph node metastases histologically and negative IHC were defined as pN0(i-). The Van Nuys classification system was used for grading the DCIS. Microinvasive disease was defined as tumor invading ≤1 mm, according to the seventh edition of the American Joint Committee on Cancer (AJCC) staging manual for breast cancer.
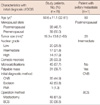
The clinicopathological characteristics such as age, menopausal state, initial diagnostic method, operation method, whether the mass was palpable, microcalcification on mammogram, and initial diagnostic method were evaluated based on medical records. Prognostic factors including whole tumor size, pathologic T stage, nodal status, nuclear grade, resection margin status, comedo necrosis, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), Bcl-2, and Ki67 were evaluated.
Patients were divided into two groups according to the consistency between initial and final diagnosis. The underestimated group consisted of patients whose diagnosis was upgraded to invasive breast carcinoma or at least microinvasion in final diagnosis. The consistent group was defined as the patients whose final diagnosis was the same as initial diagnosis.
The statistical analysis was performed using SPSS version 15.0 (SPSS Inc., Chicago, USA). The correlation between the two groups and clinicopathological features was assessed by the chi-square test or Fisher's exact test and student t-test. We calculated the cut-off value of the significant continuous variable by calculating the area under (AUC) the receiver operating characteristic (ROC) curve. A p-value of less than 0.05 was considered statistically significant.
The average age of the 78 patients with breast cancer was 50.6±11.1 years (range, 32-81 years). All patients were female, 38 (48.7%) were postmenopausal and 39 (50.0%) were premenopausal.
Of the 78 patients studied, 30 (38.5%) underwent breast conserving surgery, and 48 (61.5%) had mastectomy. Among the 66 (84.6%) patients who underwent axillary staging, nodal metastasis was present in only one patient who underwent SLNB, and she had no additional lymph node metastasis on axillary lymph node dissection. IHC stain of sentinel nodes showed pN0(i-) in all node-negative patients.
All grades and sizes of lesions were represented, although 26 of the patients had comedocarcimona. Four patients had a focus of microinvasion (<1 mm) with an initial diagnosis of DCIS. All patients were within the resection margin-free state (margin distance greater than 1 cm) and none of the DCIS patients had lymphovascular invasion.
Table 1 shows the general characteristics of the patients; 45 patients (57.7%) had microcalcification on mammogram and 31 patients (39.7%) had palpable masses on physical examination. In particular, the patient with axillary metastasis, she has a palpable tumor about 2 cm in diameter without comedo necrosis and she underwent CNB as an initial diagnostic method. Thirty-five patients (44.9%) underwent CNB and 42 patients (53.8%) underwent excison biopsy as the initial diagnostic biopsy method.
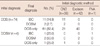
Eleven of the 35 patients (31.4%) with a CNB diagnosis of DCIS had invasive carcinoma in the surgical specimen, while patients with DCIS diagnosed by an excision biopsy as the initial diagnostic method had no invasive carcinoma in final diagnosis (Table 2).
Four patients had a CNB diagnosis of DCIS with areas of invasion <1 mm, amounting to DCISM. One of these patients (25%) had invasive carcinoma in the surgical specimen.
Of the 78 patients who had DCIS or DCISM initially, 17.9% overall were upgraded to invasive breast carcinoma or at least microinvasion in final diagnosis.
Of the 35 patients who had DCIS in the CNB specimen, the rate of underestimation was relatively high at 37.1%.
Of the 12 patients who were upgraded to the IDC in final diagnosis, 11 patients (91.7%) underwent CNB as initial diagnostic method (Table 3). In these patients, the mean size of whole tumor was 31.8±9.0 mm, but in most of these the size of invasive tumor size was less than 2 cm (83.3%). Seven of the 12 patients (58.3%) had comedo necrosis.
Compared with the consistent group between initial and final diagnosis, the underestimated group had more palpable masses (p=0.038) and a higher rate of CNB as the initial diagnostic method (p<0.001) (Table 4). Also, the underestimated group more frequently underwent mastectomy than consistent group (p=0.040). The underestimated group was statistically significantly associated tumor size, comedo necrosis, and ER status in univariate analysis (p<0.001, p=0.037, and p=0.023, respectively). No significant differences were seen in mean value of age, menopausal state, microcalcification on preoperative mammogram and nuclear grade between the two groups.
In patients with an initial diagnosis of DCIS by CNB, invasive breast cancer was statistically associated with the mean size of whole tumor and HER2 overexpression (Table 5). Logistic regression analysis was performed to assess the independent association of invasive breast cancer with statistically significant predictive factors in univariate analysis. The odds ratios of significant factors were 1.1 (95% confidence interval [CI], 1.0-1.2) and 0.3 (95% CI, 0.1-0.9) at tumor size and HER2 overexpression, respectively. We determined the ROC and AUC value for tumor size and based on the cut-off value of 2.35 cm, AUC was 0.797, sensitivity was 84.6%, and specificity was 68.2%.
DCIS is diagnosed when a proliferation of cancerous cells within the mammary ductal-lobular system without invasion, and microscopically basement membrane is preserved. According to the definition, DCIS cells do not metastasize and DCIS is not a systemic disease, therefore the need for ALND for DCIS is generally believed to be unwarranted. However, several studies investigating lymph node status in patients with DCIS reported axillary lymph node metastasis in DCIS [1-3,6,7], and the role of ALND in patients with DCIS remains controversial.
SLNB is accepted as a standard axillary approach for clinically axillary negative breast cancer patients [4,5]. The technique is highly accurate with low false-negative rates and is associated with significantly lower morbidity than ALND [18,19]. Reported rates of positive SLNB results associated with pure DCIS vary between 2% and 13% [1-3,6,7]. Although most clinicians have abandoned the routine use of SLNB in all patients with DCIS, many still believe that there is a subset of patients at high risk for microinvasive disease and subsequent axillary metastasis who may benefit from the procedure [3,11-13]. In contrast, the present study has shown that the rate of lymph node metastases in patients with DCIS is extremely low and the results of SLNB in patients with pure DCIS by excision showed no lymph node metastasis. Therefore, on this basis, to recommend SLNB for patients with DCIS seems to be inappropriate.
However, several studies demonstrated that preoperative diagnoses of DCIS based on CNB are likely to be underestimated. Rates of underestimated diagnosis range from 8.3% to 43.6% [14-17], and in this study, the rate of underestimation in patients with a CNB diagnosis as initial diagnostic method was relatively high at 37.1%. It is well known that axillary lymph node status is the most important prognostic indicator of breast cancer, and subsequent lymph node dissection will be required when invasive disease is identified in the final pathology. Many surgeons have therefore recommend SLNB for patients with DCIS who undergo mastectomy because there is no opportunity to perform lymphatic mapping after mastectomy if invasive disease is identified in the breast on final pathology review. Although routine SLNB in all patients with DCISM is not warranted, several authors have demonstrated that microinvasive disease raises the possibility of axillary metastases, and the lymph node metastases seen in final pathology may reflect the presence of small invasive lesions that went undetected in the tissue sampling process [20,21].
Because final histological type as invasive cancer was an important predictor of positive SNs, we also evaluated the clinicopathologic predictors of invasive cancer in patients with a preoperative diagnosis of DCIS or DCISM. Comparative analysis between the consistent group and the underestimated group in initial diagnosis of DCIS showed 6 independent predictors of underestimation in these patients: palpable masses on physical examination, CNB as the method of preoperative diagnosis, mastectomy as the operation method, mean size of whole tumor, comedo necrosis and ER status. The finding that larger DCIS size is more likely to be associated with invasive cancer is in agreement with the published literature [13,22,23]. In patients with DCIS, the presence of extensive calcification or of an associated mass lesion at mammography suggests a greater likelihood of an invasive component [24]. However in this study, invasive cancer was not significantly associated with the presence of microcalcification on mammogram. Some authors have also reported that on multivariate analysis, patients diagnosed with DCIS or DCISM by CNB were at increased risk for invasive cancer compared with patients diagnosed by excision biopsy [13,22,23], and our results are consistent with those previously reported.
Studies have attempted to quantify underestimation and to predict which DCIS lesions at CNB will show invasion at final excision histological examination [25-27]. In a meta-analysis of studies reporting cases of CNB diagnosis of DCIS and presenting data on DCIS underestimates, preoperative variables significantly associated with understaging include biopsy device and guidance method, size, grade, mammographic features, and palpability [28]. In our study, significant factors predictive of invasive breast cancer in patients with an initial diagnosis of DCIS by CNB were tumor size and HER2 status.
We analyzed the associated factors to a high underestimation rate of CNB. Obtaining a greater volume of tissue with larger gauge core biopsy reduces underestimation of invasive cancer [15]; limitations of sampling with a 14 gauge core biopsy device and inadequate sampling error therefore seemed to be one of the factors. Another important finding was that CNB has a much lower specificity for sampling distortions relative to other diagnostic methods [15], and that CNB does not discriminate well between invasive cancer and DCIS that have a lack of basement membrane at samples. Furthermore, most invasive cancer had invasive foci smaller than 5 mm in tumor in our data; thus the invasive component seemed to have been missed by CNB, resulting in underestimation. Some studies showed that the availability of USG guidance as an office procedure has increased the precision of the CNB considerably [28,29]. However, inadequacy is not significantly associated with guidance or core method in our data, since all lesions were sampled under USG guidance regardless of whether or not they were clinically palpable.
Our study has a few limitations, including the small cohort, possibility of missing cases of micrometastases or isolated tumor cells in sentinel nodes, and the absence of molecular study of sentinel nodes. Furthermore, we have not evaluated long-term follow-up information for our cohort. Despite these limitations, we believe that this study provides a valuable guide for physicians who treat patients with an initial diagnosis of DCIS. With these limitations in mind, we conclude that SLNB in patients with an initial diagnosis of DCIS should be limited to patients who are planned to undergo mastectomy or who have a DCIS with palpable masses on physical examination, or whose mammogram reveals microcalcification. Patients who have a CNB diagnosis of DCIS have a higher risk of invasive breast cancer on final pathologic assessment of the primary tumor; in these patients, SLNB should therefore be considered as part of their primary surgical procedure, especially in patients with tumor larger than 2.35 cm and HER2 overexpression.
In conclusion, we found that the rate of underestimation in patients with a CNB diagnosis as initial diagnostic method was 37.1%. In view of the high rate of underestimation of invasive carcinoma in patients with a CNB diagnosis of DCIS in this study, SLNB appears to be warranted in this group of patients.
Figures and Tables
References
1. Kelly TA, Kim JA, Patrick R, Grundfest S, Crowe JP. Axillary lymph node metastases in patients with a final diagnosis of ductal carcinoma in situ. Am J Surg. 2003. 186:368–370.

2. Klauber-DeMore N, Tan LK, Liberman L, Kaptain S, Fey J, Borgen P, et al. Sentinel lymph node biopsy: is it indicated in patients with high-risk ductal carcinoma-in-situ and ductal carcinoma-in-situ with microinvasion? Ann Surg Oncol. 2000. 7:636–642.

3. Cox CE, Nguyen K, Gray RJ, Salud C, Ku NN, Dupont E, et al. Importance of lymphatic mapping in ductal carcinoma in situ (DCIS): why map DCIS? Am Surg. 2001. 67:513–519.
4. Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, et al. The sentinel node in breast cancer--a multicenter validation study. N Engl J Med. 1998. 339:941–946.

5. Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003. 349:546–553.

6. Pendas S, Dauway E, Giuliano R, Ku N, Cox CE, Reintgen DS. Sentinel node biopsy in ductal carcinoma in situ patients. Ann Surg Oncol. 2000. 7:15–20.

7. Wilkie C, White L, Dupont E, Cantor A, Cox CE. An update of sentinel lymph node mapping in patients with ductal carcinoma in situ. Am J Surg. 2005. 190:563–566.

8. Tada K, Ogiya A, Kimura K, Morizono H, Iijima K, Miyagi Y, et al. Ductal carcinoma in situ and sentinel lymph node metastasis in breast cancer. World J Surg Oncol. 2010. 8:6.

9. Intra M, Rotmensz N, Veronesi P, Colleoni M, Iodice S, Paganelli G, et al. Sentinel node biopsy is not a standard procedure in ductal carcinoma in situ of the breast: the experience of the European institute of oncology on 854 patients in 10 years. Ann Surg. 2008. 247:315–319.

10. Zavagno G, Carcoforo P, Marconato R, Franchini Z, Scalco G, Burelli P, et al. Role of axillary sentinel lymph node biopsy in patients with pure ductal carcinoma in situ of the breast. BMC Cancer. 2005. 5:28.

11. Intra M, Veronesi P, Mazzarol G, Galimberti V, Luini A, Sacchini V, et al. Axillary sentinel lymph node biopsy in patients with pure ductal carcinoma in situ of the breast. Arch Surg. 2003. 138:309–313.

12. Cody HS 3rd, Van Zee KJ. Point: sentinel lymph node biopsy is indicated for patients with DCIS. J Natl Compr Canc Netw. 2003. 1:199–206.

13. Yen TW, Hunt KK, Ross MI, Mirza NQ, Babiera GV, Meric-Bernstam F, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg. 2005. 200:516–526.

14. Mittendorf EA, Arciero CA, Gutchell V, Hooke J, Shriver CD. Core biopsy diagnosis of ductal carcinoma in situ: an indication for sentinel lymph node biopsy. Curr Surg. 2005. 62:253–257.

15. Ciatto S, Houssami N, Ambrogetti D, Bianchi S, Bonardi R, Brancato B, et al. Accuracy and underestimation of malignancy of breast core needle biopsy: the Florence experience of over 4000 consecutive biopsies. Breast Cancer Res Treat. 2007. 101:291–297.

16. Houssami N, Ciatto S, Ellis I, Ambrogetti D. Underestimation of malignancy of breast core-needle biopsy: concepts and precise overall and category-specific estimates. Cancer. 2007. 109:487–495.

17. Crowe JP Jr, Rim A, Patrick RJ, Rybicki LA, Grundfest-Broniatowski SF, Kim JA, et al. Does core needle breast biopsy accurately reflect breast pathology? Surgery. 2003. 134:523–526.

18. Goyal A, Douglas-Jones A, Monypenny I, Sweetland H, Stevens G, Mansel RE. Is there a role of sentinel lymph node biopsy in ductal carcinoma in situ?: analysis of 587 cases. Breast Cancer Res Treat. 2006. 98:311–314.

19. Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006. 106:4–16.

20. Son GS, Kim TH, Um JW, Lee JB, Bae JW, Koo BH. Axillary lymph node metastasis in patients of ductal carcinoma in situ or ductal carcinoma in situ with microinvasion. J Korean Breast Cancer Soc. 2004. 7:180–184.

21. Doyle B, Al-Mudhaffer M, Kennedy MM, O'Doherty A, Flanagan F, McDermott EW, et al. Sentinel lymph node biopsy in patients with a needle core biopsy diagnosis of ductal carcinoma in situ: is it justified? J Clin Pathol. 2009. 62:534–538.

22. Dillon MF, McDermott EW, Quinn CM, O'Doherty A, O'Higgins N, Hill AD. Predictors of invasive disease in breast cancer when core biopsy demonstrates DCIS only. J Surg Oncol. 2006. 93:559–563.

23. Yi M, Krishnamurthy S, Kuerer HM, Meric-Bernstam F, Bedrosian I, Ross MI, et al. Role of primary tumor characteristics in predicting positive sentinel lymph nodes in patients with ductal carcinoma in situ or microinvasive breast cancer. Am J Surg. 2008. 196:81–87.

24. Liberman L, Abramson AF, Squires FB, Glassman JR, Morris EA, Dershaw DD. The breast imaging reporting and data system: positive predictive value of mammographic features and final assessment categories. AJR Am J Roentgenol. 1998. 171:35–40.

25. Bagnall MJ, Evans AJ, Wilson AR, Pinder SE, Denley H, Geraghty JG, et al. Predicting invasion in mammographically detected microcalcification. Clin Radiol. 2001. 56:828–832.

26. Leikola J, Heikkilä P, Pamilo M, Salmenkivi K, Von Smitten K, Leidenius M. Predicting invasion in patients with DCIS in the preoperative percutaneous biopsy. Acta Oncol. 2007. 46:798–802.

27. Lee CH, Carter D, Philpotts LE, Couce ME, Horvath LJ, Lange RC, et al. Ductal carcinoma in situ diagnosed with stereotactic core needle biopsy: can invasion be predicted? Radiology. 2000. 217:466–470.

28. Brennan ME, Turner RM, Ciatto S, Marinovich ML, French JR, Macaskill P, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011. 260:119–128.

29. Shah VI, Raju U, Chitale D, Deshpande V, Gregory N, Strand V. False-negative core needle biopsies of the breast: an analysis of clinical, radiologic, and pathologic findings in 27 concecutive cases of missed breast cancer. Cancer. 2003. 97:1824–1831.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download