Abstract
Purpose
Valid determination of HER2 status is a prerequisite to establish an adequate treatment strategy for breast cancer patients, regardless of the disease stage. The goal of this study was to examine the feasibility of the newly developed silver-enhanced in situ hybridization (SISH) technique as an alternative to fluorescence in situ hybridization (FISH) for HER2 assay in primary invasive breast cancer.
Methods
FISH and SISH for HER2 amplification were performed using tissue microarray. Both methods were used in 257 consecutive primary breast cancers.
Results
HER2 amplification was observed in 62 (23.1%) of a total of 257 breast cancers based on SISH. Of the 257 breast cancers measured using both methods, the results of the two methods were consistent in 248 (concordance, 96.5%; kappa=0.903). When we compared HER2 amplification in the primary tumor with the metastatic lymph nodes of the same patients, HER2 amplification was observed in nine cases (14.0%) out of 64 cases in which HER2 was not amplified in the primary tumors. In contrast, HER2 status was completely preserved in metastatic lymph nodes showing HER2 amplification in the primary tumor.
Recent clinical trials have shown that the addition of trastuzumab and lapatinib to standard chemotherapy improves the disease-free survival of ERBB2 (HER2)-overexpressing (HER2+) breast cancer in adjuvant settings, as well as in metastatic cancer [1-3]. The clinical benefits of targeted anti-HER2 therapy are confined to patients with HER2+ breast cancer. Thus, valid determination of HER2 status is a prerequisite for establishing adequate treatment strategies for breast cancer patients, regardless of disease stage [4].
Immunohistochemistry (IHC) is not completely accurate in determining HER2 status, especially in equivocal cases; however, it has become a widely accepted method for HER2 determination because of its practical convenience. IHC results are often hampered by various methodological heterogeneities, such as inadequate storage of tissue samples, variation in the fixation procedure, different antibodies, lack of formal training and education, and inter-observer variation despite its technological convenience [5,6].
DNA is biologically more stable than protein; thus, it is less likely to be affected by preservation conditions. Fluorescence in situ hybridization (FISH) allows the direct quantification of gene copy numbers on an individual slide and results can be more objective than IHC.
FISH has been regarded as the gold standard for HER2 status determination in breast cancer. However, FISH has not been as widely accepted as IHC in clinics because it is more time-intensive and requires special equipment, such as a fluorescence microscope and multicolor filters. Chromogenic in situ hybridization (CISH) has been introduced to overcome the practical limitations of FISH. CISH uses a simple IHC-like peroxidase reaction and, unlike FISH, does not require additional equipment [7,8]. Moreover, most pathologists are familiar with peroxidase-based immunostaining. Another advantage of CISH over FISH in routine practice is that simultaneous verification of histology can be performed using CISH. With FISH, adequate histopathological evaluation of individual cells is impossible. The recent development of dual-color probes for HER2 and CEP17 enables the identification of polysomy using CISH [9]. Silver-enhanced in situ hybridization (SISH) has been developed as an alternative method to FISH and CISH for HER2 determination [10-12]. SISH is a novel bright-field in situ hybridization technique similar to CISH. It is a fully automated system developed by Ventana Medical System (Tucson, USA), that improves the efficiency and consistency of bright-field in situ hybridization, reducing the risk of error. Automated detection of chromogenic signals also allows HER2 and CEP17 assays to be performed on consecutive tissue slides.
We performed this study to examine the feasibility of SISH as an alternative to FISH for assaying HER2 amplification in clinical breast cancer. Specifically, we compared the results from SISH with those from FISH. Additionally, we analyzed the HER2 status in primary breast cancer with metastatic cancer of the lymph nodes in the same patients.
In total, 257 primary invasive breast carcinomas were collected at Inje University Sanggye Paik Hospital, Seoul, Korea. Histopathological classification and determination of the tumor collecting regions were performed using hematoxyline and eosin (H&E) slides. Invasive ductal carcinomas were graded as 1, 2, or 3 using the Nottingham histological grading system [13], in ascending degree of malignancy. This study was conducted under the approval of the Institutional Review Board of Inje University Sanggye Paik Hospital.
Recipient blocks were made with purified agar in 3.8×2.2 cm frames. Holes (2 mm) were made on the recipient blocks using a core needle and the agar core was discarded. Donor blocks were prepared after thorough evaluation of the H&E slides. Three case cores were obtained separately from each primary breast cancer sample and metastatic lymph node specimen. Representative cancer portions taken from matching donor blocks were transplanted to the recipient blocks using a 2-mm core needle. Recipient blocks were framed in a mold that is used to frame conventional paraffin blocks, and paraffin was then added to the frame. Consecutive 4.0-µm thick sections were cut from the recipient blocks using an adhesive-coated slide system (Instrumedics Inc., Hackensack, USA) supporting the cohesion of the 2-mm array elements on the glass [8].
For HER2 IHC, the FDA-approved HercepTest™ (DAKO, Carpinteria, USA) was used manually according to the manufacturer's protocol. Prior to staining, tissue slides were deparaffinized and rehydrated. For epitope retrieval, the slides were soaked in a heated water bath at 95-99℃ for 40 minutes. A peroxidase-blocking reagent (100 µL) was applied for 5 minutes. Then, 100 µL of anti-HER2 protein reagent was added and the slides were incubated for 30 minutes. A visualization reagent (100 µL) was applied for 30 minutes. A substrate-chromogen solution (diaminobenzidine) was then added and the color was developed for 10 minutes. The slides were counterstained by immersion in a bath of hematoxylin for 2-5 minutes.
IHC staining was semiquantitatively evaluated using the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines. Staining results were scored as 0 (negative; no staining), 1 (negative; faint/barely perceptible incomplete membrane staining), 2 (equivocal; weak to moderate complete membrane staining in >10% of the tumor cells or strong, complete membrane staining in <30% of the tumor cells), and 3 (positive; strong complete membrane staining in >30% of the tumor cells) [4]. Positive and negative controls were used to validate each assay run.
Prior to in situ hybridization, tissue slides were deparaffinized and incubated in a SPOT-Light Heat Pretreatment buffer (Zymed Inc., South San Francisco, USA) at 92-100℃ for 15 minutes. After washing with phosphate buffer saline (PBS), 100 mL of SPOT-Light Tissue Pretreatment Enzyme (Zymed) was applied at 37℃ for 5 minutes. The slides were washed with PBS and dehydrated with graded concentrations of ethanol. A coverslip was placed on the slide after application of 15 µL of digoxigenin-labeled HER2/neu probe. The slides were placed on a 95℃ hot plate for 5-10 minutes and were then incubated at 37℃ for 16-24 hours. After incubation, the slides were treated with 0.5× sodium chloride citrate for 5 minutes and then washed with PBS/Tween solution. The slides were treated with 3% hydrogen peroxide for 10 minutes and 100 µL of FITC-sheep anti-digoxigenin (Zymed) was then applied for 30-60 minutes. After washing with PBS, 100 µL of HRP-goat anti-FITC (Zymed) was applied for 30-60 minutes. After washing again with PBS, 150 µL of 3,3-diaminobenzidine tetrahydrochloride was applied for 20-30 minutes. The slides were counterstained with H&E after washing with purified water and dehydrated with ethanol and xylene. Amplification of HER2 was defined when the gene copy number was more than four or when a large copy cluster was seen in more than 50% of cancer cell nuclei.
HER2 SISH was performed on an automated instrument, a Ventana Benchmark (Ventana Medical Systems), according to the manufacturer's protocols for INFORM HER2 DNA and chromosome 17 probes. Testing for the HER2 gene and chromosome 17 was performed on sequential sections. Two sections were baked at 60℃ for 20 minutes. The HER2 DNA probe was denatured at 95℃ for 12 minutes and hybridization was performed at 52℃ for 2 hours. The chromosome 17 probe was denatured at 95℃ for 12 minutes and hybridization was performed at 44℃ for 2 hours. After hybridization, appropriate stringency washes were performed three times at 72℃ for the HER2 probe and three times at 59℃ for the chromosome 17 probe. Both DNP-labeled probes were visualized using a rabbit anti-DNP primary antibody and the ultraView SISH Detection Kit (Ventana). The slides were counterstained with hematoxylin for examination by light microscopy.
Evaluation of HER2 gene amplification status was performed in a blind manner using the ASCO/CAP guidelines [4]. HER2/CEP 17 SISH signals were selected and 20 nonoverlapping nuclei were analyzed. The ratio of HER2/CEP 17 was then calculated. A ratio of <1.8 indicated that HER2 gene was not amplified, whereas a ratio of >2.2 indicated amplification of the gene. The equivocal range was defined as HER2/CEP 17 ratios from 1.8 to 2.2. For the equivocal cases, signals from 20 further tumor nuclei were counted in a second target area and a new ratio was calculated. Benign breast epithelial cells and other adjacent benign cells were used as internal controls.
Consecutive sections (4 µm) were cut from the paraffine blocks. Before hybridization, the sections were deparaffinized, air dried, and dehydrated in 100% ethanol after incubation at 56℃ for 24 hours. The slides were treated with a wash buffer (Abbott Laboratories, Abbott Park, USA) for 3 minutes after treatment with 0.2 N HCl for 20 minutes. A pretreatment solution (Abbott) was applied for 30 minutes at 80℃ and the slides were then washed with purified water. The slides were serially treated twice with a wash buffer at 45-50℃ and air dried. The slides were fixed in 10% buffered formalin for 10 minutes and then washed with wash buffer at 45-50℃. For denaturation, the slides were immersed in a denaturation solution (Abbott) for 5 minutes at 72℃ followed by serial dehydration with 70%, 85%, and 100% ethanol at 45-50℃. For hybridization, a 10 µL LSI HER-2/CEP17 probe (PathVysion™; Abbott) was applied and a coverslip was placed over the probe. After overnight hybridization at 37℃ in a humidified chamber, the slides were washed at 72℃ with a post-hybridization wash buffer (Abbott) for 2 minutes. Nuclei were counterstained with 10 µL 4,6-diamino-2-phenylindole (Abbott). Centromere 17 (CEP) and HER2 copy numbers were estimated for the predominant tumor cell populations.
Following the ASCO/CAP guidelines, at least 20 malignant nonoverlapping nuclei were selected from two different areas in each patient, and the HER2/CEP 17 ratio was calculated [4]. Amplification was scored as negative (ratio <1.8) or positive (ratio >2.2) for gene amplification. The equivocal range was defined as HER2/CEP 17 ratios from 1.8 to 2.2. For the equivocal cases, signals from 20 further tumor nuclei were counted in a second target area and a new ratio was calculated. Benign breast epithelial cells and other adjacent benign cells were used as the internal control.
Results from CISH, SISH, and FISH were merged and analyzed. A chi-squared test was used for data analysis. Correlations between the results were estimated using Spearman's correlation coefficients (kappa). A kappa value of 1 denotes complete agreement, values more than 0.75 denote excellent agreement, values between 0.4 and 0.75 denote fairly good agreement, and values less than 0.4 denote poor agreement. Statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, USA). Statistical significance was defined as a p-value of less than 0.05.
HER2 amplification measured by SISH was observed in 62 cases (24.1%) of the 257 studied breast cancers (Figures 1, 2). In contrast, the frequency of HER2 amplification was 64 cases by CISH (24.9%) and 61 cases (23.7%) by FISH. The median patient age was 46 (range, 31-82) years. The estrogen receptor (ER) was expressed in 160 patients (62.3%). Lymph node metastasis was present in 143 patients (55.6%). When we analyzed the HER2 status according to the clinical and pathological characteristics of the patients, HER2 amplification was significantly increased in both high grade (p<0.05) and ER-negative tumors (p<0.05).
We compared the results from SISH with those from CISH and FISH. Results of SISH and FISH were consistent in 248 of the 257 tumors. The concordance between the two methods was 96.5% (kappa=0.903). Results from SISH and CISH were consistent in 247 tumors, with a concordance rate of 96.2%. HER2 amplification was detected by CISH in 6 cases, although the amplification signals were not observed by SISH in these cases. Of these 6 cases, polysomy was observed in 3 (50.0%) (Table 1).
HER2 protein expression was analyzed using a HercepTest™ and we compared these results with HER2 amplification status, measured by SISH, CISH, and FISH (Table 2). In 43 cases with HercepTest 3+ results, HER2 amplification was confirmed in 42 cases (97.7%). Of the 189 tumors with HercepTest 0-1+ results, HER2 amplification was observed in 6 (3.1%) by FISH, 3 (1.6%) by SISH and 7 (3.7%) by CISH. In contrast, HER2 amplification was confirmed in 52-68% by FISH, SISH, and CISH (Table 2).
We compared HER2 amplification in the primary tumor with metastatic lymph nodes of the same patients. Eighty-seven out of the 143 cases were available to evaluate the HER2 gene status in metastatic lymph nodes. Of the 87 cases, HER2 amplification was observed in 9 cases (14.0%) in which HER2 was not amplified in the main tumors. In contrast, HER2 status was completely preserved in metastatic lymph nodes in which HER2 was amplified in the primary tumor (Table 3).
We used the INFORM HER2 SISH technology developed by Ventana Medical Systems (Tucson, USA) in the current study. SISH was more rapidly performed than FISH, taking only 6 hours. When we analyze DNA amplification using FISH, overnight hybridization is usually required. Furthermore, SISH requires only a conventional light microscope, making its use possible for routine pathology laboratories.
The current study indicates that the results obtained with SISH for HER2 amplification were consistent with those of FISH, showing a high concordance, of 96.5%. According to the ASCO/CAP guidelines, more than 95% concordance should be achieved to validate novel ISH technology or IHC procedures [4]. Results of the current study are comparable with the accuracy of other studies using SISH for HER2 assay [14,15]. The two studies that compared SISH with FISH reported a concordance rate of 94-98%.
The overall frequency of HER2 amplification ranges between 12% and 20% for primary breast cancers [16,17]. Identification of proper candidates for anti-HER2 treatment is the most important step to maximize treatment efficacy while minimizing unnecessary costs due to improper patient selection. Some patients who will not benefit from anti-HER2 treatment may be exposed to unnecessary treatment due to inaccurate identification. In contrast, some patients may lose the opportunity to receive more effective and less toxic target therapy. However, uncertainty remains regarding the best approach for selecting patients for HER2 treatment. There is also uncertainty regarding the most appropriate and efficient testing strategy and the reliability and interpretation of the HER2 test results.
While FISH is the gold standard for the HER2 assay, it is not always available, even in developed countries. The main obstacles to the wide acceptance of FISH are high costs and labor-intensiveness due to the requirement of specialized instruments and the typical lack of quality control systems in medium- or small-volume institutions. However, FISH is currently performed in centralized and qualified centers, even in large multicenter clinical trials [18-20]. Nevertheless access to FISH testing and reluctance to export tissue samples are other barriers to centralized FISH testing. For this reason, IHC remains the most popular HER2 assaying method, despite its inferiority to FISH.
Based on the current study along with another report, SISH could be a reasonable alternative to FISH for HER2 status determination because of its practical convenience for clinical breast cancer testing. Education for standardization and quality control is significantly less complicated for SISH than for FISH because of its technical simplicity. Moreover SISH has an advantage in that stained samples can be stored at room temperature and do not readily decay over time.
Another type of bright-field in situ hybridization for HER2 is CISH. We previously reported CISH results for HER2 amplification with 188 consecutive breast cancers, which were compared with results obtained by FISH using the same tissue microarray [8]. Concordance between CISH and FISH was 94% in that report, which is comparable to the results of the current study showing concordance between SISH and FISH. In the current study, we performed CISH with the same tissue array blocks. CISH results were almost identical to those obtained by FISH and SISH. SISH and CISH may be practical alternatives to FISH in situations in which FISH is not available for the HER2 assay. However, the previous single-color CISH determined the HER2 gene and we could not identify polysomy. CISH is not yet considered an alternative choice in the selection of patients to be treated with trastuzumab.
Discordance of HER2 amplification between primary breast cancer and metastatic lesions, including axillary lymph nodes, has been extensively investigated. In the current study, we identified 9 discordant cases out of 87 matched samples. Although most data suggest good overall concordance between primary and metastatic lesions [21,22], some data have demonstrated discordance in up to 20% of cases [23]. Whether HER2 status is maintained in distant metastatic lesions remains controversial. A recent study reported that a positive conversion of HER2 status was observed in a significant proportion of metastatic lesions [24]. In that study, HER2 status was discordant between primary tumors and metastatic sites in 127 of a total of 382 cases. The investigators reported that positive conversion of HER2 was observed in 37 cases. However, HER2 status was only measured by immunohistochemistry in that study, thus the validity of the study was hampered by 2+ cases. In the current study, HER2 status was not accurate by immunohistochemistry in 2+ cases. Furthermore in the current study, HER2 amplification was observed in 52-68% of HercepTest 2+ cases by FISH, SISH, and CISH.
The wide range of variation in HER2 conversion between primary tumor and metastatic lesions stems from the different time intervals and metastatic sites among studies. Our results suggest that a positive conversion of HER2 status in synchronous metastatic lymph nodes is not rare. We previously reported that HER2 status between primary tumors and corresponding lymph nodes were concordant in 93% of cases [25]. In that study, we used immunohistochemistry alone for HER2 assay in the lymph nodes. A concordance of 90% is similar to that observed in the current study. However, HER2 was not overexpressed in the metastatic lymph node, while the primary tumor showed HER2 overexpression and amplification in 5 cases. This result somewhat differs from that of the current study.
We hypothesize that discordance of HER2 amplification between primary tumors and metastatic sites could increase with increasing time intervals due to the de novo conversion of HER2 status. Clonal outgrowth with genetic modification might influence the discordant HER2 status in metastatic sites with increasing time intervals and adjuvant systemic therapy. This might contribute to the relatively lower frequency of discordance observed in our study. The high concordance of HER2 status between invasive and intraductal components of individual tumors from our previous study [25] may shed light on the relatively low incidence of positive HER2 status conversion in the current study. In 270 consecutive breast cancers, HER2 amplification was consistent between the invasive and intraductal components of 266 individual tumors in our previous study [25].
Assay methods might influence the lower frequency of discordance, because most studies used IHC for HER2 assay [21,22,24]. At this point, we cannot conclude whether de novo conversion of HER2 amplification occurs in a synchronous or metachronous pattern.
SISH showed accuracy comparable with that of FISH with respect to HER2 status determination in formalin-fixed paraffin-embedded tissues. SISH may be a viable alternative to FISH due to its practical convenience in a clinical situation where FISH is not available. HER2 amplification in metastatic axillary lymph nodes coincided well with that of primary tumors. However, discordance between primary tumors and metastatic axillary lymph nodes was observed in a considerable proportion of studied breast cancers. In this situation, negative conversion of HER2 amplification was rare whereas positive conversion in axillary lymph nodes was common.
In node-positive breast cancers, as well as in recurrent or metastatic settings, HER2 status confirmation in metastatic nodes, regardless of HER2 status in primary tumors, appears to be necessary for proper management. This can provide the opportunity for less toxic anti-HER2 biologic treatments to a significant proportion of breast cancer patients who otherwise are not candidates for anti-HER2 biologic treatment. Thus, the results of this study will contribute to improved clinical outcomes in breast cancer.
Figures and Tables
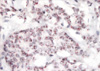 | Figure 1Silver-enhanced in situ hybridization revealed two HER2 black dots and two chromosome 17 red dots in each breast cancer cell (×400). |
References
1. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005. 353:1673–1684.

2. Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007. 369:29–36.

3. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006. 355:2733–2743.

4. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007. 25:118–145.

5. Penault-Llorca F, Bilous M, Dowsett M, Hanna W, Osamura RY, Rüschoff J, et al. Emerging technologies for assessing HER2 amplification. Am J Clin Pathol. 2009. 132:539–548.

6. Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009. 14:320–368.

7. Tanner M, Gancberg D, Di Leo A, Larsimont D, Rouas G, Piccart MJ, et al. Chromogenic in situ hybridization: a practical alternative for fluorescence in situ hybridization to detect HER-2/neu oncogene amplification in archival breast cancer samples. Am J Pathol. 2000. 157:1467–1472.
8. Park K, Kim J, Lim S, Han S, Lee JY. Comparing fluorescence in situ hybridization and chromogenic in situ hybridization methods to determine the HER2/neu status in primary breast carcinoma using tissue microarray. Mod Pathol. 2003. 16:937–943.

9. Laakso M, Tanner M, Isola J. Dual-colour chromogenic in situ hybridization for testing of HER-2 oncogene amplification in archival breast tumours. J Pathol. 2006. 210:3–9.

10. Powell RD, Pettay JD, Powell WC, Roche PC, Grogan TM, Hainfeld JF, et al. Metallographic in situ hybridization. Hum Pathol. 2007. 38:1145–1159.

11. Kang J, Kwon GY, Lee YH, Gong G. Comparison of silver-enhanced in situ hybridization and fluorescence in situ hybridization for HER2 gene status in breast carcinomas. J Breast Cancer. 2009. 12:235–240.

12. Kim TJ, Kim TE, Jung ES, Yim HW, Song BJ, Jung SS, et al. The comparison of automated silver in situ hybridization and fluorescence in situ hybridization for evaluating HER2 gene amplification in breast carcinoma. J Breast Cancer. 2009. 12:295–301.

13. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991. 19:403–410.

14. Dietel M, Ellis IO, Höfler H, Kreipe H, Moch H, Dankof A, et al. Comparison of automated silver enhanced in situ hybridization (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists. Virchows Arch. 2007. 451:19–25.

15. Papouchado BG, Myles J, Lloyd RV, Stoler M, Oliveira AM, Downs-Kelly E, et al. Silver in situ hybridization (SISH) for determination of HER2 gene status in breast carcinoma: comparison with FISH and assessment of interobserver reproducibility. Am J Surg Pathol. 2010. 34:767–776.

16. Grimm EE, Schmidt RA, Swanson PE, Dintzis SM, Allison KH. Achieving 95% cross-methodological concordance in HER2 testing: causes and implications of discordant cases. Am J Clin Pathol. 2010. 134:284–292.

17. Ross JS. Human epidermal growth factor receptor 2 testing in 2010: does chromosome 17 centromere copy number make any difference? J Clin Oncol. 2010. 28:4293–4295.

18. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005. 353:1659–1672.
19. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005. 353:1673–1684.

20. Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007. 369:29–36.

21. Shimizu C, Fukutomi T, Tsuda H, Akashi-Tanaka S, Watanabe T, Nanasawa T, et al. c-erbB-2 protein overexpression and p53 immunoreaction in primary and recurrent breast cancer tissues. J Surg Oncol. 2000. 73:17–20.

22. Tanner M, Järvinen P, Isola J. Amplification of HER-2/neu and topoisomerase IIalpha in primary and metastatic breast cancer. Cancer Res. 2001. 61:5345–5348.
23. Santinelli A, Pisa E, Stramazzotti D, Fabris G. HER-2 status discrepancy between primary breast cancer and metastatic sites. Impact on target therapy. Int J Cancer. 2008. 122:999–1004.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download