Abstract
Acinic cell carcinoma (ACC) of the breast is extremely rare and is characterized by widespread acinar cell-like differentiation. We report of a 39-year-old woman presented with a palpable breast mass with significant morphological, immunohistochemical and ultrastructural findings. Histologically, ACC showed a diffuse glandular infiltrative pattern, with small acinar or glandular structures mixed with solid nests. Neoplastic cells were monotonous proliferation of cells with a granular or clear cytoplasm, resembling acinar cells of the salivary glands or Paneth cells. Both glandular and solid tumor cell populations were strongly positive for lysozyme and α-1-antitrypsin.
Acinic cell carcinoma (ACC) of the breast with features of acinar-type differentiation was first described by Roncaroli et al. [1] in 1996 as the breast counterpart of identical tumors of the parotid gland. Since then, several cases of acinar cell-like breast carcinoma have been reported [1-5]. These reported carcinomas show diffuse infiltrative growth patterns of small glandular structures and are composed of cells with a coarse granular or clear cytoplasm resembling acinar cells of the salivary glands or Paneth cells.
We report a case of pure ACC of the breast with characteristic histopathological findings, immunohistochemical expression, and ultrastructural zymogen granules in a 39-year-old woman. The tumor is cytologically, immunohistochemically and ultrastructurally very similar to cases of ACC of the salivary gland. Our differential diagnosis with microglandular adenosis (MGA), and other carcinomas showing granular cytoplasm is discussed. Reported herein is the first case of pure ACC of the breast in Korea.
A 39-year-old woman presented with a 1-year history of a palpable breast mass. A fine-needle aspiration (FNA) of the mass was performed under the initial clinical diagnosis of benign fibroadenoma. No abnormalities were discovered on physical examination. The FNA result was atypical cytology, and showed scattered or rarely clustered, uniformly round cells with small nuclei and a moderate amount of cytoplasm, suggesting a benign or low grade malignant tumor. Sonography and mammographic findings showed no evidence of abnormality in either breast or axillae. The breast lesion was treated with a lumpectomy.
On gross examination, the tumor was a 5.5×3.0 cm, ill defined, gray-brown with a rubbery consistency, and a slightly lobulated appearance (Figure 1). Microscopically, the tumor showed a diffuse infiltrative growth patterns with small acinar or glandular structures mixed with solid nests (Figure 2). Most of the tumor was comprised of monotonous round cells with a finely granular, weakly eosinophilic, or clear vacuolated cytoplasm resembling acinar cells of the salivary glands. Morphologically distinct cells showed dark eosinophilic coarse granules resembling Paneth cells (Figures 3, 4). Some neoplastic cells had a clear cytoplasm. The lumen of the small acinar or glandular structure sometimes contained red colloid-like material and microcalcifications. Lymphatic invasion was often observed. Other solid nests similar to in situ and invasive ductal carcinoma were focally observed. These large solid tumor cell nests revealed central comedo-like necrosis, reminiscent of ductal carcinoma in situ (Figure 5). However, these cells were also cytologically and immunohistochemically closely similar to typical acinar cells. The mitotic count was high in areas with more solid nests (up to 5-35/high power field), but lower in areas with acinar cells (up to 0-3/high power field). The surgical margin was focally positive for tumor cells. Periodic acid-Schiff (PAS) stain demonstrated strong staining of cytoplasmic granules with diastase resistance.
Immunohistochemically, both the glandular and solid tumor cell populations were strongly positive for lysozyme (Figure 6A), α-1-antitrypsin, and epithelial membrane antigen (EMA). Staining showed complete circumferential intense staining for E cadherin (Figure 6B) and focal cytoplasmic reactions for the S-100 protein (Figure 6C). Chromogranin was also expressed focally in the areas of the granular acinar cells. Focal neoplastic cells were weakly positive for cytokeratin (CK) 7 (Figure 6D), compared to a strongly positive reaction in normal ducts and lobules. The other areas were negative for estrogen receptor, progesterone receptor, gross cystic disease fluid protein of 15 Kda (GCDFP 15), cytokeratin 20, Muc2, Muc5A, Muc6, neuron specific enolase, CD68, smooth muscle actin, and human epidermal growth factor receptor-2 (HER2/neu) (Table 1).
Electron microscopy performed with formalin-fixed tissue demonstrated numerous variable sized electron dense granules in the cytoplasm that were consistent with zymogen granules, and a prominent, rough endoplasmic reticulum (Figure 7). The patient was transferred to an another hospital for a breast conserving operation with a sentinel node biopsy and axillary node dissection. Only one non-sentinel node revealed metastatic carcinoma, measuring 0.3 cm at the greatest dimension. All remaining nodes were free of tumor cells.
It is well known that breast and salivary gland tissues share embryologic similarities [2]. Similar to the salivary glands, the breast has modified sweat or apocrine glands, and some tumors arising in the breast are subject to development of salivary type tumors, such as pleomorphic adenoma, adenoid cystic carcinoma, adenomyoepithelioma, and ACC. Matoso et al. [2] emphasized that breast tumors with salivary gland differentiation originate from a malignant transformation of terminal duct-lobular units with metaplastic changes. Furthermore, the immunohistochemical profile of the tumor reported herein was identical to that of salivary ACC [3,4]. The significance of acinar cell differentiation in breast carcinomas is not clear. Lysozyme can be detected in mammary epithelium during lactation and lactating lobules share a similar immunophenotype with breast ACC [3].
Previous reports have shown that the pathogenesis of ACC is related to MGA or secretory carcinoma (SC) [3,5,6]. However, these reports noted no relationship between MGA or SC and ACC based on a lack of ETV6 rearrangement in ACC [6]. Therefore, ACC is a distinct entity other than one variant or a related lesion [5].
Peintinger et al. [7] described the differential points of MGA, pointing out that ACC of the breast has a microglandular growth pattern, luminal eosinophilic colloid-like secretory materials, and diffuse and intense positive immunoreactivity for the S-100 protein. These features are similar to carcinoma arising in MGA. However, ACC shows immunoreactivity for EMA, lysozyme, amylase, and α-1-antichymotrypsin. While the glands in MGA are surrounded by a basal lamina, the neoplastic glands of ACC do not possess a basal lamina.
Microscopically, ACC of the breast reveals two morphologically distinct cell populations focally merging into one another [3,4,7,8]. The present tumor showed a diffuse infiltrative growth pattern with small acinar or glandular structures mixed with solid nests (Figure 2). The solid nests, commonly observed in in situ and invasive ductal carcinoma, were focally recognized in the present case. These large solid tumor cell nests showed central comedo-like necrosis, which is reminiscent of ductal carcinoma in situ (Figure 5). This feature was initially interpreted as ordinary invasive ductal carcinoma. However, these cells were also cytologically and immunohistochemically closely similar to typical acinar cells. Both glandular and solid tumor cell populations were strongly positive for lysozyme (Figure 6A), α-1-antitrypsin, and EMA. The tumor cells were negative for GCDFP 15, estrogen receptor, progesterone receptor, and HER2/neu. Re-evaluation of the present case led to a diagnosis of pure ACC, due to the immunohistochemical results. And special stains in this case showed results similar to a previously described tumors [7,8]. Damiani et al. [3] studied acinar cell differentiation in salivary gland tumors and noted the presence of zymogen-type granules. Zymogen is only one of the components in ACC, as amylase, lysozyme and α-1-antichymotrypsin are also constituents of salivary gland acinar cells. They found amylase expression in all breast tumors studied, as well as in all studied cases of ACC of the parotid gland. However, amylase was negative in ordinary breast carcinomas.
In this case, electron microscopy demonstrated numerous variable sized electron dense zymogen granules in the cytoplasm, that were consistent with acinar cell granules. Intense immunohistochemical expression of lysozyme and α-1-antitrypsin combined with ultrastructural findings of numerous zymogen granules confirmed the diagnosis of ACC of the breast.
Damiani et al. [3] described six patients with ACC of the breast. All patients were 35-80 years (mean-age, 56 years). The tumor size ranged from 2 to 5 cm. All patients were treated with surgery. Axillary dissection was performed in three cases, and lymph node metastases were found in two. Of the six patients, one was alive and well after 5 years, three were alive and well after 1 year, one had a recurrence after 4 years, and one was lost to follow-up. These cases demonstrate that primary ACC of the breast can be associated with a poor prognosis, particularly when the tumor is high grade with additional adverse prognostic features. The present case was a high grade invasive solid tumor with a high mitotic count for small limited foci. Therefore, the prognosis of this case might be unfavorable. Peintinger et al. [7] suggested that ACC of the breast belongs to a group of clinically low-grade malignant tumors, even with some unfavorable prognostic parameters such as high mitotic activity and steroid hormone receptor negativity.
In summary, this case was histologically, immunohistochemically, and ultrastructurally typical of pure ACC of the breast.
Figures and Tables
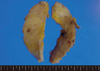 | Figure 1The tumor was a 5.5×3.0 cm, ill defined mass, with a gray brown rubbery consistency and a slightly lobulated appearance. |
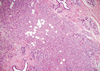 | Figure 2Tumor cells growing in diffuse infiltrative patterns with small acinar or glandular structures and large solid nests in a fibro-fatty stroma and a normal mammary parenchyma (H&E stain, ×40). |
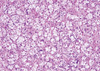 | Figure 3Tumor cells were characterized by monotonous round cells with a finely granular, weakly eosinophilic, or clearly vacuolated cytoplasm and resembled acinic cells of the salivary glands (H&E stain, ×200). |
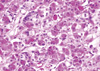 | Figure 4Tumor cells showing dark eosinophilic coarse granules, resembling Paneth cells. Nuclei were irregular, and some intranuclear inclusions were noted (H&E stain, ×200). |
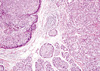 | Figure 5Large solid nests of tumor cells revealed central comedo-like necrosis, reminiscent of ductal carcinoma in situ (H&E stain, ×100). |
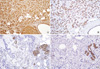 | Figure 6Immunohistochemical staining shows (A) strong immunoreactivity to lysozyme, (B) complete circumferential intense staining for E cadherin, (C) focal cytoplasmic reaction to S-100 protein, and (D) weak positivity for CK 7 in the luminal membrane of tumor cells and a strong positive reaction in normal ducts (A-C, ×100; D, ×200). |
References
1. Roncaroli F, Lamovec J, Zidar A, Eusebi V. Acinic cell-like carcinoma of the breast. Virchows Arch. 1996. 429:69–74.

2. Matoso A, Easley SE, Gnepp DR, Mangray S. Salivary gland acinar-like differentiation of the breast. Histopathology. 2009. 54:262–263.

3. Damiani S, Pasquinelli G, Lamovec J, Peterse JL, Eusebi V. Acinic cell carcinoma of the breast: an immunohistochemical and ultrastructural study. Virchows Arch. 2000. 437:74–81.

4. Tanahashi C, Yabuki S, Akamine N, Yatabe Y, Ichihara S. Pure acinic cell carcinoma of the breast in an 80-year-old Japanese woman. Pathol Int. 2007. 57:43–46.

5. Hirokawa M, Sugihara K, Sai T, Monobe Y, Kudo H, Sano N, et al. Secretory carcinoma of the breast: a tumour analogous to salivary gland acinic cell carcinoma? Histopathology. 2002. 40:223–229.

6. Reis-Filho JS, Natrajan R, Vatcheva R, Lambros MB, Marchió C, Mahler-Araújo B, et al. Is acinic cell carcinoma a variant of secretory carcinoma? A FISH study using ETV6'split apart' probes. Histopathology. 2008. 52:840–846.

7. Peintinger F, Leibl S, Reitsamer R, Moinfar F. Primary acinic cell carcinoma of the breast: a case report with long-term follow-up and review of the literature. Histopathology. 2004. 45:645–648.

8. Elster EA, Markusic J, Ball R, Soballe P, Henry M, Louie A, et al. Primary acinic cell carcinoma of the breast. Am Surg. 2002. 68:993–995.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download