Abstract
A primary fibrosarcoma of the breast is a rare tumor. Here we report on a case of a primary fibrosarcoma of the breast that presented as a palpable left breast mass in a 47-year-old woman. The physical examination revealed a 3 cm sized, round mass in the left upper outer breast. The mammograms revealed a 3 cm sized, partially circumscribed and partially obscured, high density mass in the upper outer quadrant of the left breast. An ultrasonogram demonstrated a 3 cm sized, ovoid, circumscribed and hypoechoic mass with peripheral increased vascularity on Doppler imaging. Surgical excision was performed and the pathology revealed a low grade fibrosarcoma.
Primary sarcomas of the breast are a heterogeneous group of malignant mesenchymal neoplasms. Fibrosarcoma of the breast is included in this group of sarcomas and this tumor is always malignant, although the malignancy may vary in degree [1,2].
Primary breast fibrosarcoma is a rare neoplasm. In a review of the literature, Barnes and Pietruszka [1] reviewed all the cases of breast sarcoma that were seen at the Health Center Hospitals of the University of Pittsburgh during the 1945-1976 period, and they found that only 10 cases of primary breast sarcoma had been reported. Further, Adem et al. [3] reported that primary breast sarcoma comprised only 0.0006% of all breast malignancies presenting at his institution from 1940 to 1999. However, the exact incidence of primary breast fibrosarcoma is not known nor has it been reported in the literature.
Reports in the medical literature with the radiologic imaging of primary breast fibrosarcomas are also very rare. To the best of our knowledge, there has been no prior report of the sonographic imaging of primary fibrosarcoma of the breast in the English medical literature. Here we report on the mammographic and sonographic imaging and pathological findings of a low grade, primary breast fibrosarcoma.
A 47-year-old woman presented with a painless palpable mass in her left breast. The patient incidentally detected the mass 10 days prior to the clinical visit. She had no history of breast abnormalities, previous breast surgery or radiation therapy. A physical examination revealed a 3 cm size, relatively well-defined mass in the upper outer quadrant of the left breast. There was no overlying skin abnormality or palpable axillary or supraclavicular lymph nodes.
The mammograms showed a 3 cm, ovoid, partially obscured, partially circumscribed, and high-density mass without internal calcifications in the upper outer quadrant of the left breast (Figure 1A, B). A sonogram showed a 2.5 cm, ovoid, circumscribed and homogeneous hypoechoic mass with posterior acoustic enhancement at the 2 o'clock site of the left breast (Figure 1C) and color Doppler image showed vascularity in the peripheral portion of the mass (Figure 1D). We therefore classified the mass as category 4b according to the Breast Imaging Reporting and Data System (BI-RADS).
We then performed an ultrasonographic-guided core needle biopsy, and the histopathological examination of the biopsy specimen revealed the presence of a cellular, spindle cell proliferative neoplasm with intermittent mitosis. These findings did not allow differentiation between spindle cell mesenchymal neoplasm and fibroepithelial neoplasm, and the findings could not provide a diagnosis. Therefore, surgical excision was performed, and the gross specimen showed a firm, fleshy, well circumscribed, round mass that was gray-white and it measured 3.5 cm at the greatest dimension. There was no intratumoral necrosis. Histopathological examination showed a high cellular, spindle cell tumor that displayed an interdigitating fasciculated growth pattern, the so-called herringbone pattern (Figure 2A). The tumor cells were fusiform or spindle shaped cells that varied little in size and shape, and they had scanty cytoplasm with indistinct cell borders. The nuclei showed mild to moderate atypia. The mitotic activity was up to 9-10 per 10 HPF (Figure 2B). The negative immunostaining results for S-100 protein, smooth muscle actin and many kinds of keratin, including CK AE1/AE3, high molecular weight CK, low molecular weight CK, CK7 and epithelial membrane antigen, distinguished this tumor from spindle cell malignant melanoma or malignant peripheral nerve sheath tumors, leiomyosarcoma and metaplastic carcinoma (Figure 2C). The final diagnosis was a low-grade fibrosarcoma. She had no postoperative complications, and she remains well without signs of local recurrence or distant metastases 10 months following her surgery.
Pure stromal sarcoma of the breast includes fibrosarcoma, malignant fibrous histiocytoma, liposarcoma, rhabdomyosarcoma, leiomyosarcoma, hemangiosarcoma, malignant schwannoma, osteogenic sarcoma and chondrosarcoma [1]. Adult fibrosarcoma is a malignant tumor that is composed of fibroblasts with variable degrees of collagen production and in classical cases a herringbone architecture, according to the World Health Organization [4]. Fibrosarcomas can occur anywhere in the body, but they usually occur primarily in the extremities. However, primary sarcomas also occur the breast or in the pectoral fascia.
The overall incidence of primary fibrosarcoma of the breast has not been established. Pollard et al. [5] reviewed only 25 cases of primary breast sarcoma that were diagnosed pathologically at a London hospital during an 80-year period. Among these, there were only 4 cases of fibrosarcoma. The peak incidence of primary breast sarcoma occurs in the fifth and sixth decades of life [6]. For fibrosarcoma of the breast, the peak age of incidence is unknown. However, Roberson [2] has reviewed the literature and evaluated 49 reported patients with fibrosarcoma of the breast, and half of them were between the ages of 41 and 60. These patients usually present with a complaint of a lump in one breast of two to six months duration and pain was present in over one-third of the cases. In contrast, the clinical presentation of a common primary breast sarcoma is a painless breast lump [3].
The size of primary breast sarcomas is variable and it ranges from less than 1 cm to larger than 40 cm [6]. Primary breast sarcomas may spread by direct invasion or hematogenous metastasis. Axillary lymph node involvement is very rare [7]. And the incidence of actual node metastasis also appears to be very low [2]. The prognosis is usually based on the size of the tumor and the histological grade [3].
Terrier et al. [8] retrospectively reviewed 33 cases of primary breast sarcoma. They assessed the prognostic factors of primary breast sarcoma. Seventeen cases were cystosarcomas phyllodes and 16 cases were stromal sarcomas. The stromal sarcomas were classified as malignant fibrous histiocytomas (11 cases), fibrosarcomas (2 cases), leiomyosarcomas (2 cases) and liposarcoma (1 case). In that study, the metastasis-free survival rate was significantly correlated only with the histological grade, which consisted of the tumor differentiation, the presence of tumor necrosis and the mitotic activity. The clinical courses and survival of the cystosarcoma and stromal groups were identical, which raises questions about the clinical value of this pathologic distinction. All the local recurrence, metastasis or death occurred within 30 months, although the follow-up was much longer. Performing immunohistochemistry has been disappointing for identifying the specific histologic sub-types.
Blanchard et al. [9] retrospectively reviewed 55 patients with a primary breast sarcoma. Of the 55 patients, 17 had breast-conserving therapy and 38 women had mastectomy. The mean patient age at presentation was 52 years (range, 22 to 82 years). The types of sarcoma included angiosarcoma (18), malignant fibrous histiocytoma (11), stromal sarcoma (8), liposarcoma, leiomyosarcoma, dermatofibrosarcoma protuberans (4), osteosarcoma (3), fibrosarcoma (2) and rhabdomyosarcoma (1). Follow-up information was available for 53 patients with a mean follow-up of 81 months. Twenty-nine of the 53 patients (55%) developed recurrent sarcoma and 23 patients (43%) died of their disease. Twenty-seven patients had no evidence of recurrence, and 3 patients were alive with disease at the last follow-up. The overall median survival of the patients with breast sarcoma was 58 months. The patients with angiosarcoma had a poorer outcome than did the other sarcoma patients. Twelve of 18 patients (67%) died of angiosarcoma, compared with 11 of the 32 patients (34%) with all the other types of sarcomas combined. Of the 34 patients who did not receive adjuvant chemotherapy or radiation, 13 died of their disease (38%), as compared with 10 of the 16 patients (63%) who did receive adjuvant therapy. In that study, adjuvant chemotherapy and radiation did not improve survival. Surgical extirpation remains the only effective treatment [9].
Elson et al. [10] reported the mammographic findings of 5 cases with fibrosarcoma of the breast. In their study, the mammograms showed high-density masses with margins varying from poorly defined to well-defined and the diameters ranged from 1.5 cm to 7.0 cm. Calcified osseous elements were present in 1 of the masses. However, these findings were nonspecific. In the case presented here, the mass appeared as an ovoid, partially obscured and partially circumscribed, high-density mass on mammography and this is also a nonspecific finding and it could not distinguish fibrosarcoma from other breast carcinomas. On ultrasonography, the mass appeared as an ovoid, circumscribed and homogeneous hypoechoic solid mass with posterior acoustic enhancement.
In our patient, the size of the tumor was 3 cm and there was no lymph node or distant metastasis. The tumor stage was a 1A according to the American Joint Committee on Cancer [11]. According to the National Comprehensive Cancer Network clinical practice guidelines in oncology, surgery is the primary treatment for low-grade stage 1 tumors and it is considered definitive if the tumor free margins are greater than 1 cm or the fascia plane is intact. For surveillance, stage I tumors are routinely followed with taking the recent medical history and conducting a physical examination every 3-6 months for 2-3 years and then annually. Chest imaging should also be considered every 6 to 12 months. Because these patients' risk never returns to zero, long-term follow-up is indicated and this should include MRI or CT scanning. If the final tumor-free resection margins is 1 cm or less, then radiotherapy should be considered [12]. In our case, our patient was treated by surgical excision with a 1 cm negative surgical margin. Radiotherapy was not performed. Local recurrence and distant metastasis did not occur in our patient 10 months after surgery.
In conclusion, fibrosarcoma of the breast is a rare tumor and the imaging results make it difficult to differentiate this type of lesion from other malignant masses.
Figures and Tables
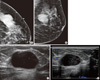 | Figure 1Imaging findings. (A, B) 47-year-old woman resented with a palpable mass in the left breast. Cranicaudal (A) and mediolateral oblique views (B) of a mammogram show an ovoid, partially circumscribed and partially obscured, high-density mass in the upper outer quadrant of the left breast. (C) An ultrasonogram shows an ovoid circumscribed, and homogenous hypoechoic mass with posterior acoustic enhancement in the left breast. (D) The Doppler ultrasonogram shows vascularity in the peripheral portion of the mass. |
 | Figure 2Microscopic findings. (A) Microscopic findings shows spindle cell tumor that has a cell pattern of columns of short parallel lines with all the lines in one column sloping one way and lines in adjacent columns sloping the other way (herring bone pattern) (H&E stain, ×400). (B) Microscopic findings show celluar spindle cells with elongated nuclei and mild to moderate cytologic atypia arranged in interwoven bundles and intermittent mitotic figures (arrow) (H&E stain, ×400). (C) Immunohistochemical finding shows negative reaction to smooth muscle actin immunostain on spindle tumor cells (smooth muscle actin Immunostain, ×400). |
References
1. Barnes L, Pietruszka M. Sarcomas of the breast: a clinicopathologic analysis of ten cases. Cancer. 1977. 40:1577–1585.

2. Roberson GV. Fibrosarcoma of the breast. J Ark Med Soc. 1973. 69:257–265.
3. Adem C, Reynolds C, Ingle JN, Nascimento AG. Primary breast sarcoma: clinicopathologic series from the Mayo Clinic and review of the literature. Br J Cancer. 2004. 91:237–241.

4. Fletcher CD, Unni KK, Mertens F. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. 2002. Lyon: IARC Press.
5. Pollard SG, Marks PV, Temple LN, Thompson HH. Breast sarcoma. A clinicopathologic review of 25 cases. Cancer. 1990. 66:941–944.

6. Trent II JC 2nd, Benjamin RS, Valero V. Primary Soft Tissue Sarcoma of the Breast. Curr Treat Options Oncol. 2001. 2:169–176. McDivitt R, Stewart FW, Berg JW. Tumours of the breast. In: Atlas of Tumor Pathology. Washington, D.C.: Armed Forces Institute of Pathology; 1968. p.127-30.

7. Hefny AF, Bashir MO, Joshi S, Branicki FJ, Abu-Zidan FM. Stromal sarcoma of the breast: a case report. Asian J Surg. 2004. 27:339–341. Moore MP, Kinne DW. Breast sarcoma. Surg Clin North Am 1996;76: 383-92.

8. Terrier P, Terrier-Lacombe MJ, Mouriesse H, Friedman S, Spielmann M, Contesso G. Primary breast sarcoma: a review of 33 cases with immunohistochemistry and prognostic factors. Breast Cancer Res Treat. 1989. 13:39–48.

9. Blanchard DK, Reynolds CA, Grant CS, Donohue JH. Primary nonphylloides breast sarcomas. Am J Surg. 2003. 186:359–361.

10. Elson BC, Ikeda DM, Andersson I, Wattsgård C. Fibrosarcoma of the breast: mammographic findings in five cases. AJR Am J Roentgenol. 1992. 158:993–995.

11. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 2010. 7th ed. New York: Springer.
12. Clinical practice guidelines in oncology - v.2.2010: soft tissue sarcoma. National Comprehensive Cancer Network. Accessed June 23rd, 2010.
http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download