Abstract
Purpose
αB-crystallin, a small heat shock protein, is an anti-apoptotic protein associated with aggressive tumor behavior. A recent study revealed that αB-crystallin is overexpressed in a metastatic variant of the GI101A human breast carcinoma cell line. The purpose of this study was to investigate whether αB-crystallin is related to other breast tumor markers and can predict a breast cancer prognosis.
Methods
Eighty-two patients who underwent breast cancer surgery at Hallym Sacred Heart Hospital were enrolled. αB-crystallin expression was determined by immunohistochemical staining. Estrogen receptor, progesterone receptor (PR), human epidermal growth factor receptor, lymphovascular invasion, histological grade, other tumor markers and time to recurrence were compared with αB-crystallin expression.
Results
αB-crystallin expression in breast cancer tissues was associated with PR (p=0.030), the number of metastatic lymph nodes (pN) (p=0.020), lymphovascular invasion (p=0.022), histological grade (p=0.004) and triple negative breast cancer (TNBC) (p=0.004). αB-crystallin expression significantly decreased time to recurrence (p=0.039).
Conclusion
The results revealed a strong relationship between αB-crystallin and poor prognostic factors such as the number of metastatic lymph nodes (especially pN2), TNBC, and rapid time to recurrence. We believe that αB-crystallin could be a novel oncoprotein biomarker of a poor prognosis in breast cancer.
Many studies have attempted to identify predictors of a poor prognosis for breast cancer. Breast cancer can be classified into five major subtypes based on gene expression signature; luminal A, luminal B, normal breast-like, human epidermal growth factor receptor (HER2), and basal-like. Among these, basal-like breast cancer (BLBC) is associated with a poor prognosis, because these cancers are highly proliferative and invasive, and they metastasize rapidly to the lung and brain [1]. The molecular classification of breast cancer has provided new prognostic factors. One of the molecules related to prognosis is αB-crystallin, which was thought to be associated with a poor prognosis in many studies. αB-crystallin is a member of the conserved small heat shock protein and is expressed in diverse malignancies. Crystallins, including αB-crystallin, are soluble proteins found primarily in the lens of the eye, and αB-crystallin is found in normal and diseased non-lenticular tissue [2]. Indeed, αB-crystallin has been found in malignant diseases such as gliomas, renal cell carcinomas, and breast carcinomas [3-5], and its expression correlates with poor clinical outcomes in breast and head and neck carcinomas [5-7]. Recent studies have indicated that αB-crystallin is expressed in BLBCs and likely contributes to their aggressive phenotype [8]. But, it is unknown whether αB-crystallin overexpression is driven by promoter transactivation, loss of transcriptional inhibition, increased DNA copy number, or by other means, such as a mutation of promoter elements [7]. αB-crystallin influences cytoprotective effects by functioning as a molecular chaperone to inhibit intracellular protein aggregation. Additionally, αB-crystallin inhibits apoptosis in response to many different stimuli, including chemotherapy drugs, tumor necrosis factor-α, tumor necrosis factor-related apoptosis-inducing ligand, and reactive oxygen species through the cell death protease caspase-3 and by preventing the mitochondrial translocation of proapoptotic Bcl-2 family members such as Box [2-6].
A recent study indicated that αB-crystallin is expressed in BLBCs and predicts poor survival independent of tumor grade, lymph node metastases, estrogen receptor (ER) or HER2 status [7]. Furthermore, αB-crystallin is expressed more in breast cancers with lymph node metastasis [5]. Although the anti-apoptotic function of αB-crystallin is thought to be related to such a poor breast cancer prognosis, clinical studies are insufficient.
The objective of this study was to investigate the correlation between αB-crystallin expression and established prognostic factors such as molecular subtypes, histological grade, and lymph node metastasis. Furthermore we wanted to determine whether αB-crystallin is a novel predictor of aggressive breast cancer.
Eighty-two invasive ductal carcinomas (IDC) were obtained from surgical resections conducted at the Department of Surgery at Hallym Sacred Heart Hospital from August 2002 to June 2006. Ipsilateral axillary lymph node dissection was performed in all cases. All samples were paraffin-embedded, and whole tissue sections were previously fixed in 10% neutral buffered formalin or an alcoholic formalin mixture. Clinicopathological factors were evaluated, including age at initial diagnosis, tumor size, lymph node metastasis, lymphovascular invasion, histological grade, and tumor markers such as ER, progesterone receptor (PR), and HER2. The histological grade was assessed by a modified Bloom-Richardson-Scarff grading system. Tumor marker positivity was evaluated based on pathology reports. We considered HER2 staining scores of 2 and 3 as HER2 positive.
Hematoxylin and eosin tissue sections were reviewed by a pathologist, who selected areas of invasive tumor to be placed on a tissue microarrary, for each case included in the study. Five µm thick sections were cut and placed on a tissue microarray.
Slides were incubated for 30 minutes, deparaffinized, and rinsed. Heat antigen unmasking was performed for 20 minutes, followed by the addition of primary antibody (1:200, anti-αB-crystallin) for 1 hour at room temperature. After washing, the secondary antibody was added for 30 minutes at room temperature. αB-crystallin immunohistochemistry was performed using a commercially available monoclonal antibody to αB-crystallin (1:200 in antibody diluents, SPA-222; Stressgen Biotechnologies, Victoria, Canada).
Cytoplasmic expression of αB-crystallin was scored using a four-tiered system. Staining was graded as follows: 0, negative staining; 1, weakly positive staining; 2, moderately positive staining; 3, highly positive staining in cytoplasm (Figure 1). αB-crystallin expression was analyzed according to various clinical and biological characteristics such as tumor size, lymph node metastasis, lymphovascular invasion, histological grade, tumor markers such as ER, PR, HER2, and time to recurrence.
DBSTAT software version 4.1 (DBSTAT Co., Seoul, Korea) was used. Correlations between αB-crystallin and clinicopathological characteristics were assessed using chi-square and Fisher's exact tests. Time to recurrence between the αB-crystallin positive and negative groups was analyzed by the Kaplan-Meier method. A p<0.05 was considered significant.
Patient age ranged from 28-76 years with a median age of 53 years. The mean follow-up period was 50 months. All of the pathological types were IDC. Nine cases of distant metastasis to bone, lung, liver, adrenal gland and the leptomenix were found.
Twenty nine tumors (35.4%) had no cytoplasmic αB-crystallin staining (score 0), 23 (28.0%) had weakly positive staining (score 1), 15 (18.3%) had moderate staining (score 2), and 15 tumors (18.3%) had strong cytoplasmic staining (score 3). We defined scores of 0 and 1 as being indicative of "negative or low expression" and scores of 2 and 3 as being indicative of "positive or high expression." As a result, 52 tumors (63.4%) had low expression, and 30 tumors (36.6%) had high expression.
αB-crystallin was not correlated with tumor size (p=0.602), lymph node status (p=0.403), or distant metastasis (p=0.064). However, it was correlated with histological grade (p=0.004) and lymphovascular invasion (p=0.022). No statistical correlation was found between αB-crystallin score and lymph node metastasis (Figure 2). Six of nine cases who developed distant metastasis positively expressed αB-crystallin. Although the patient population was small and the p value was >0.05, αB-crystallin expression tended to have a marginal association with distant metastasis. Furthermore, when lymph node status was classified into pN stages by the number of metastatic lymph nodes, αB-crystallin was expressed more strongly in pN2 than in pN0 or pN1 (p=0.020) (Figure 3).
αB-crystallin expression was associated with PR (p=0.030) and triple negative breast cancer (TNBC) (p=0.004). A total of 60% of the TNBCs were αB-crystallin positive, whereas 26.8% of non-TNBCs were αB-crystallin positive. Other factors such as ER, HER2, cytokeratin 5/6 (CK 5/6), and epidermal growth factor receptor (EGFR) had no association with αB-crystallin expression (Table 2).
The difference in the time to distant metastasis between patients who were αB-crystallin negative and positive was assessed by the Kaplan-Meier method using the log-lank test. αB-crystallin expression significantly decreased time to distant metastasis. A statistical significance was found between any type of distant metastasis and αB-crystallin expression (p=0.039) (Figure 4).
Clinical indices such as tumor size, grade and axillary lymph node metastasis are useful prognostic factors in breast cancer. Among these factors, axillary lymph node metastasis is the most important prognostic factor for patients with breast cancer [9]. Many other studies have been conducted to identify predictors related to axillary lymph node status. However, no factor accurately predicts axillary lymph node metastasis.
Recently, Chelouche-Lev et al. [5] found significantly more αB-crystallin-positive tumors among patients with lymph node-positive disease than patients with lymph node-negative disease (p<0.001). They reported that constitutive αB-crystallin expression in human breast cancer cells in vitro was associated with the ability to metastasize in nude mice, and that the highest expression levels were observed in cell lines established from metastatic cells. Similarly, αB-crystallin staining was significantly associated with lymph node metastasis in our study. However, in this study, αB-crystallin was expressed significantly more in the pN2 breast cancer group than in pN0 or pN1 groups, which was different from Chelouche-Lev's study. We thought that the differences in the patient cohorts made it difficult to compare the results of Chelouche-Lev et al. with ours. Although αB-crystallin seemed to be associated with lymph node metastasis, we do not think αB-crystallin is an accurate enough factor to eliminate axillary lymph node dissection. We thought that other factors co-expressed with αB-crystallin such as stromal cell-derived factor-1 and its receptor, CXC chemokine receptor 4 [10] need to be studied to accurately predict prognosis and survival.
Additionally, 60% of TNBCs were αB-crystallin positive, whereas 26.8% of non-TNBCs were αB-crystallin positive. αB-crystallin may be associated with TNBC. Patients with a TNBC had significantly shorter survival following the first metastatic event than those with a non-TNBC [11,12]. As most BLBCs are ER-negative and HER2-negative, the term TNBC has previously been substituted for BLBCs [13]. Although there is overlap between TNBC and BLBC, 76% of BLBCs expressed either EGFR or CK5/6, and these markers define BLBCs [14]. Both gene expression data and a recent immunohistochemistry analysis of breast cancer tissue have suggested an association between αB-crystallin and BLBCs [15]. The gene expression data revealed that αB-crystallin is included in the basal-like gene cluster [5,16,17]. αB-crystallin is commonly expressed in BLBCs and is thought to be a sensitive (81%) and specific (100%) marker for BLBCs [18]. These studies provide additional independent validation linking αB-crystallin to BLBCs [18]. We did not find a relationship between BLBC and αB-crystallin because of insufficient immunohistochemistry staining for CK5/6 and EGFR.
It seems that αB-crystallin is resistant to neoadjuvant chemotherapy. Ivanov et al. [19] reported that αB-crystallin-positive tumors had poorer overall response rates than αB-crystallin-negative tumors (clinical overall response rate, 21% vs. 59%, respectively, p=0.005; overall pathological response rate, 16% vs. 70%, respectively, p<0.001).
Despite the pathogenic significance of αB-crystallin, the regulatory mechanism of its expression related to aggressiveness is poorly understood. Heat-shock proteins such as αB-crystallin play a major role in the ability of in vitro tumor cells to overcome stress caused by external stimuli, and enhance resistance to apoptosis [20,21]. Such resistance may result from the partial binding of αB-crystallin to caspase-3 and the resulting inhibition of the autoproteolytic maturation of caspase-3, a key effector molecule in the apoptotic cascades [22]. These findings suggest that the anti-apoptotic effect of αB-crystallin may be related to the aggressiveness of breast cancer. Furthermore, recent research shows that Ets1, an oncogenic transcription factor, binds to the αB-crystallin promoter and regulates its expression through an ETS-binding site dependent mechanism. Ets1 overexpression in breast cancer cells increases αB-crystallin protein level, whereas silencing Ets1 reduces αB-crystallin levels. Moreover, Ets1 is expressed in BLBC and is associated with poor survival [8]. The rapid time to distant metastasis in our study may be linked to these in vitro findings.
Consistent with earlier studies, we demonstrated that αB-crystallin expression was associated with poor prognosis such as axillary lymph node metastasis in pN2, TNBC, and rapid time to recurrence. We think that αB-crystallin could be used as an oncoprotein to predict poor clinical outcomes. However, further studies are needed to prospectively elucidate the role of this novel tumor marker as a clinical prognostic factor in breast cancer.
Figures and Tables
 | Figure 1αB-crystallin expression scoring. Staining was graded as follows: 0, negative staining; 1, weakly positive staining; 2, moderately positive staining; 3, highly positive staining (Immunohistochemical staining for αB-crystallin, ×100).
(A) Score=0, (B) Score=1, (C) Score=2, (D) Score=3.
|
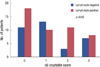 | Figure 2αB-crystallin score distributions among patients with lymph node negative and lymph node positive breast cancer. No statistical correlation was found between αB-crystallin score and each group. |
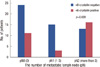 | Figure 3αB-crystallin expression according to the number of metastatic lymph nodes (pN stage). αB-crystallin expression in breast cancers was expressed more strongly in pN2 than in pN0 and pN1 (p=0.006). |
 | Figure 4Time to distant metastasis based on αB-crystallin expression. αB-crystallin expression significantly decreased time to distant metastasis. Statistical significance was found between any type of distant metastasis and αB-crystallin expression (p=0.039). |
References
1. Yehiely F, Moyano JV, Evans JR, Nielsen TO, Cryns VL. Deconstructing the molecular portrait of basal-like breast cancer. Trends Mol Med. 2006. 12:537–544.

2. Pinder SE, Balsitis M, Ellis IO, Landon M, Mayer RJ, Lowe J. The expression of alpha B-crystallin in epithelial tumours: a useful tumour marker? J Pathol. 1994. 174:209–215.

3. Mehlen P, Mehlen A, Guillet D, Preville X, Arrigo AP. Tumor necrosis factor-alpha induces changes in the phosphorylation, cellular localization, and oligomerization of human hsp27, a stress protein that confers cellular resistance to this cytokine. J Cell Biochem. 1995. 58:248–259.

4. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001. 98:10869–10874.

5. Chelouche-Lev D, Kluger HM, Berger AJ, Rimm DL, Price JE. AlphaB-crystallin as a marker of lymph node involvement in breast carcinoma. Cancer. 2004. 100:2543–2548.

6. Chin D, Boyle GM, Williams RM, Ferguson K, Pandeya N, Pedley J, et al. Alpha B-crystallin, a new independent marker for poor prognosis in head and neck cancer. Laryngoscope. 2005. 115:1239–1242.

7. Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, et al. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006. 116:261–270.

8. Bosman JD, Yehiely F, Evans JR, Cryns VL. Regulation of alphaB-crystallin gene expression by the transcription factor Ets1 in breast cancer. Breast Cancer Res Treat. 2010. 119:63–70.

9. Fisher ER, Anderson S, Redmond C, Fisher B. Pathologic findings from the National Surgical Adjuvant Breast Project protocol B-06. 10-year pathologic and clinical prognostic discriminants. Cancer. 1993. 71:2507–2514.

10. Kim JO, Suh KS, Lee DH, Sul HJ, Lee JU, Song KS. Expression of CXCR4 and SDF-1alpha in primary breast cancers and metastatic lymph nodes. J Breast Cancer. 2009. 12:249–256.

11. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007. 13(15 Pt 1):4429–4434.

12. Tischkowitz M, Brunet JS, Bégin LR, Huntsman DG, Cheang MC, Akslen LA, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007. 7:134.

13. Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007. 13:2329–2334.

14. Rakha EA, Tan DS, Foulkes WD, Ellis IO, Tutt A, Nielsen TO, et al. Are triple-negative tumours and basal-like breast cancer synonymous? Breast Cancer Res. 2007. 9:404.

15. Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004. 10:5367–5374.

16. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000. 406:747–752.

17. Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003. 100:8418–8423.

18. Sitterding SM, Wiseman WR, Schiller CL, Luan C, Chen F, Moyano JV, et al. AlphaB-crystallin: a novel marker of invasive basal-like and metaplastic breast carcinomas. Ann Diagn Pathol. 2008. 12:33–40.

19. Ivanov O, Chen F, Wiley EL, Keswani A, Diaz LK, Memmel HC, et al. AlphaB-crystallin is a novel predictor of resistance to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2008. 111:411–417.

20. Parcellier A, Gurbuxani S, Schmitt E, Solary E, Garrido C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun. 2003. 304:505–512.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download