Abstract
Purpose
Fulvestrant, a potent estrogen receptor (ER) antagonist with a novel mechanism of action, has shown efficacy in pretreated patients with advanced breast cancer. We assessed the efficacy and tolerability of fulvestrant in Korean postmenopausal women.
Methods
Of the 25 candidates identified at Asan Medical Center, Seoul, Korea, six were deemed ineligible due to inadequate baseline and follow-up imaging. The 19 patients included in this retrospective analysis received the approved dose of fulvestrant (250 mg intramuscular injection, once per month) as second- (n=8), third- (n=7), or fourth-line (n=4) endocrine therapy.
Results
At a median follow-up of 7.4 months (range, 1.2-34.8 months), the 19 patients received a median of four cycles (range, 1-34 cycles) of fulvestrant. Median time to progression was 5.5 months (95% confidence interval [CI], 0.4-10.7 months), and median overall survival was 17.9 months (95% CI, 2.7-33.1 months). Among 17 evaluable patients, one (5.3%) achieved a partial response, 10 (52.6%) showed stable disease, and six (31.6%) showed progressive disease. The clinical benefit rate was 26.3%. Four patients (21.1%) reported adverse events, but all were grade 1 or 2.
Endocrine therapy is a standard treatment option in postmenopausal women with hormone receptor-positive advanced breast cancer and has been preferred due to its generally favorable toxicity profiles. Several endocrine therapy agents with different mechanisms of actions are currently available, including the selective estrogen receptor modulators tamoxifen and toremifene, the non-steroidal aromatase inhibitors anastrozole and letrozole, and the steroidal aromatase inactivator exemestane.
Fulvestrant is a potent estrogen receptor (ER) antagonist with a novel mechanism of action. Due to its steroidal structure, fulvestrant completely inhibits ER signaling by blocking and degrading the ER protein [1]. In contrast to tamoxifen, fulvestrant has no demonstrable agonist activity [2]. Furthermore, preclinical studies have shown a lack of cross-resistance between fulvestrant and tamoxifen [3].
Clinical trials of fulvestrant in postmenopausal women with hormone receptor-positive advanced breast cancer have found that fulvestrant was at least as effective as anastrozole in tamoxifen-resistant patients [4,5] and was at least as effective as exemestane in patients previously treated with non-steroidal aromatase inhibitors [6]. Additionally, several retrospective studies have found that fulvestrant is effective and tolerated in postmenopausal women with ER-positive advanced breast cancer [7-9]. However, little is known about the efficacy and safety profiles of fulvestrant in Asian populations. Therefore, we retrospectively assessed the clinical efficacy and tolerability of fulvestrant in Korean patients with hormone receptor-positive advanced breast cancer.
A retrospective review of patients with advanced breast cancer treated at Asan Medical Center, Seoul, Korea between April 2007 and October 2009 identified 25 patients, each of whom received at least one dose of fulvestrant. Data regarding baseline characteristics, previous treatments, response to prior endocrine therapy and survival were obtained by reviewing patient's medical records. However, six patients were excluded due to inadequate baseline and/or follow-up imaging assessment, which may have biased the results. Hormone receptor status was assessed immunohistochemically. Human epidermal growth factor receptor 2 (HER2/neu) was considered positive if immunoreactivity was 3+ or unequivocal amplification was demonstrated by fluorescence in situ hybridization (FISH). This study was approved by the Asan Medical Center Institutional Review Board (2006-0449).
Fulvestrant (250 mg) was injected intramuscularly into the buttock every 4 weeks until disease progression. During the fulvestrant treatment period, patients underwent a physical examination and laboratory tests, including complete blood counts and chemistry every month and an imaging evaluation, such as computed tomography or magnetic resonance imaging every 2-3 months or whenever disease progression was clinically suspected. Response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 [10]. Fulvestrant was deemed to show clinical benefits if patients achieved complete response (CR), partial response (PR), or stable disease (SD) that was maintained for at least 6 months. Adverse events were graded according to the NCI-CTCAE version 3.0 [11].
Time to progression (TTP) was defined as the time from the start of fulvestrant treatment to disease progression or death from any cause or was censored at last follow-up visit. Overall survival (OS) was defined as the time from the start of fulvestrant treatment to death from any cause or was censored at the time of the last follow-up visit in patients who survived. Survival probability was estimated using the Kaplan-Meier method. The log-rank test was used to assess the association between survival outcomes and patient baseline characteristics. Hazard ratios (HR) and 95% confidence intervals (CIs) were estimated using the Cox proportional hazard regression model. A two-sided p<0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, USA).
The baseline characteristics of the 19 patients included in this analysis are summarized in Table 1. The median patient age was 55 years, and all were postmenopausal. The most common metastatic site was bone (n=13, 68.4%), and 11 (57.9%) patients had visceral organ involvement. Patients had been previously treated with a median of two (range, 1-3) endocrine therapy regimens, and 18 (94.7%) were treated with letrozole. Tamoxifen was administered to two patients in a palliative setting, but had already been used in 15 patients as an adjuvant treatment for early breast cancer. Two patients had received gonadotropin releasing hormone agonist (GnRH-agonist) as they were premenopausal. The clinical benefit of prior endocrine therapy, defined as achievement of CR, PR, or SD for at least 6 months, was observed in 13 (68.4%) patients. Prior to fulvestrant, 10 (52.6%) patients received palliative chemotherapy, with all 19 patients receiving a median of one (range, 0-6) prior regimens. Seven (36.8%) patients underwent radiotherapy for recurrent or metastatic disease. Details regarding baseline characteristics and treatment outcomes are presented individually in Table 2. Almost half of these patients were unfit or refused further chemotherapy when they were enrolled. In subset of hormone receptor and HER2/neu positive comprising 5-10% of whole breast cancer population, combined use of endocrine and HER2 target agent is promising and effective at the moment. In practice, trastuzumab is reimbursed in Korea only when it is used in combination with taxane in first line of metastatic setting or after progression beyond anthracyclines and taxane. Out of ten patients with HER2/neu positive disease, only two patients had received trastuzumab and taxane in 1st line of metastatic breast cancer (Case No. 11 and 16) before fulvestrant. The rest were either unfit for combined trastuzumab and taxane regimen or refused upfront use of trastuzumab monotherapy because of reimbursement issue.
At a median follow-up period of 7.4 months (range, 1.2-34.8 months), the 19 patients received a median of four cycles (range, 1-34 cycles) of fulvestrant. The median TTP was 5.5 months (95% CI, 0.4-10.7 months) and the median OS was 17.9 months (95% CI, 2.7-33.1 months; Figure 1). In patients who received fulvestrant as second-line endocrine therapy, the median TTP was not reached, and median OS was 15.9 months (95% CI, 0.0-39.1 months). In patients who received fulvestrant as third- or fourth-line treatment, the median TTP was 2.5 months (95% CI, 0.9-4.1 months), and the median OS was 22.0 months (95% CI, 0.0-46.7 months). Although TTP was significantly longer in patients receiving fulvestrant as second-line therapy than in those receiving it as third- or fourth-line therapy (p=0.04), OS did not differ between these two groups (p=0.87). The response to fulvestrant was evaluable in 17 patients (89.5%). One patient (5.3%) achieved PR, 10 (52.6%) achieved SD, and six (31.6%) showed PD. A clinical benefit (≥SD over 6 months) was achieved in five (26.3%) patients. The patient who achieved PR was a 48-year-old woman with chemotherapy-induced menopause, as shown by her last menstrual history and levels of hormones such as follicle stimulating hormone (FSH) and estradiol prior to the fulvestrant administration. However, during treatment with fulvestrant her serum FSH and estradiol profile restored to the premenopausal status. Despite this, she showed a stable PR for 3 months.
Our retrospective analysis showed that the approved dose (250 mg every 4 weeks) of fulvestrant was effective and well tolerated in Korean patients with advanced breast cancer who had been previously treated with endocrine and chemotherapeutic agents. All but one patient treated with GnRH agonist showed tumor progression during their previous treatment with non-steroidal aromatase inhibitors, and the 19 patients had received a median of two prior endocrine therapy regimens. Our efficacy results were consistent with the findings of previous prospective and retrospective studies [6-9]. We found that the objective response rate to fulvestrant was 5.3%, and the clinical benefit rate was 26.3%. The median TTP was 5.5 months, and the median OS was 17.9 months. In a large randomized phase III trial (EFFECT study), which showed that fulvestrant and exemestane had similar efficacy in patients with non-steroidal aromatase inhibitor-resistant advanced breast cancer, the overall response rate to fulvestrant was 7.4%, the clinical benefit rate was 32.2%, and the median TTP was 3.7 months [6]. Previous retrospective reports based on a single-center experience showed objective response rates ranging from 9.3% to 21.7%, clinical benefit rates ranging from 38.9% to 69.5%, and median TTP ranging from 4 months to 6.4 months [7-9]. Therefore, our findings suggest that fulvestrant is similarly effective in postmenopausal, heavily pretreated Korean women. Although 11 of the 19 patients in this study received fulvestrant in a third- or fourth-line setting, the survival outcomes were better than those reported for the EFFECT trial; this may be due to our small sample size, ethnic differences, differences in patient demographics, and continued administration of chemotherapy after progression while on fulvestrant.
Previous clinical trials have shown that fulvestrant is well tolerated, with toxicity profiles similar to those of other endocrine therapeutic agents. In contrast to these earlier trials, we observed no adverse events associated with the intramuscular injection, such as injection-site pain or reactions [4,6,12,13]. All adverse events related to fulvestrant were mild and tolerable.
Although fulvestrant is a novel and potent ER downregulator and has shown comparable outcomes in second- and third-line settings [6,14], first-line fulvestrant failed to show superiority to tamoxifen [13]. However, a recent multicenter, open-label, phase II study suggested that high-dose (500 mg once a month) fulvestrant may prolong disease control [12]. Despite preclinical promises and an ideal mechanism of action, fulvestrant has shown lower than expected activity in postmenopausal women with advanced breast cancer. Pharmacokinetic and pharmacodynamic studies have suggested that the approved dose (250 mg) results in a low steady-state concentration and insufficient ER downregulation [15]. The efficacy of fulvestrant may be enhanced by various doses and schedules, such as a loading dose (500 mg on day 0, 250 mg on day 14, thereafter 250 mg once per month) and higher monthly doses, both of which have shown clinically significant outcomes [6,12,16]. The therapeutic potential of fulvestrant may therefore be enhanced by investigations into its optimal dose and schedule.
Little has been reported to date about the efficacy and safety profiles of fulvestrant in Asian patients. In Korea, fulvestrant has been approved for postmenopausal women with advanced breast cancer who have progressed on tamoxifen or non-steroidal aromatase inhibitors. However, the cost of fulvestrant is not covered by national insurance; thus, limiting its use and resulting in a lack of data in Korean patients. We found that fulvestrant was also effective in a premenopausal patient, supporting the previous finding that high dose of 750 mg fulvestrant needed to reduce the effects of estrogen in premenopausal women with ER-positive breast cancer [17]. Due to the predominance of premenopausal disease in Asian populations, in contrast to Western countries [18], further clinical trials with fulvestrant are required in premenopausal as well as postmenopausal Asian women with ER-positive breast cancer.
In conclusion, fulvestrant had modest activity and a favorable safety profile in heavily pretreated Korean postmenopausal women with advanced breast cancer. Although the sample size was too small to suggest new findings based on the results, our findings provide further evidence for the use of fulvestrant in heavily pretreated patients. To improve the management of patients with ER-positive advanced breast cancer, further investigations to assess the optimal dosage of fulvestrant and to maximize its therapeutic efficacy in the cascade of endocrine therapeutic agents are warranted.
Figures and Tables
Table 2
Patient characteristics and treatment outcome
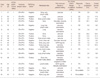
ECOG=Eastern Cooperative Oncology Group; ER=estrogen receptor; PR=progesterone receptor; PR=partial response; SD=stable disease; PD=progressive disease; GnRH-agonist=gonadotropin releasing hormone agonist.
*Immunoreactivity 3+ or 2+ with unequivocal amplification by fluorescence in situ hybridization.
Notes
References
1. Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer. 2004. 90:Suppl 1. S2–S6.

2. Addo S, Yates RA, Laight A. A phase I trial to assess the pharmacology of the new oestrogen receptor antagonist fulvestrant on the endometrium in healthy postmenopausal volunteers. Br J Cancer. 2002. 87:1354–1359.

3. Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, McCue BL, Wakeling AE, McClelland RA, et al. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst. 1995. 87:746–750.

4. Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002. 20:3386–3395.

5. Howell A, Robertson JF, Quaresma Albano J, Aschermannova A, Mauriac L, Kleeberg UR, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002. 20:3396–3403.

6. Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008. 26:1664–1670.

7. Mlineritsch B, Psenak O, Mayer P, Moik M, Namberger K, Hauser-Kronberger C, et al. Fulvestrant ('Faslodex') in heavily pretreated postmenopausal patients with advanced breast cancer: single centre clinical experience from the compassionate use programme. Breast Cancer Res Treat. 2007. 106:105–112.

8. Steger GG, Bartsch R, Wenzel C, Pluschnig U, Hussian D, Sevelda U, et al. Fulvestrant ('Faslodex') in pre-treated patients with advanced breast cancer: a single-centre experience. Eur J Cancer. 2005. 41:2655–2661.

9. Safra T, Greenberg J, Ron IG, Ben-Yosef R, Inbar M, Sarid D, et al. Fulvestrant in heavily pretreated metastatic breast cancer: is it still effective as a very advanced line of treatment? Isr Med Assoc J. 2008. 10:339–343.
10. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.
11. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003. 13:176–181.

12. Robertson JF, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, Macpherson E, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009. 27:4530–4535.

13. Howell A, Robertson JF, Abram P, Lichinitser MR, Elledge R, Bajetta E, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol. 2004. 22:1605–1613.

14. Howell A, Pippen J, Elledge RM, Mauriac L, Vergote I, Jones SE, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma: a prospectively planned combined survival analysis of two multicenter trials. Cancer. 2005. 104:236–239.

15. Robertson JF. Fulvestrant (Faslodex): how to make a good drug better. Oncologist. 2007. 12:774–784.
16. Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko I, Khasanov R, et al. Confirm: a phase III, randomized, parallel-group trial comparing fulvestrant 250 mg vs fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. Cancer Res. 2009. 69:24 Suppl. Abstract #25.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download