Abstract
Purpose
Triple receptor-negative (TRN) breast cancer is associated with high risk of recurrence and poor prognosis. The present study assessed the clinicopathologic characteristics and ultrasound (US) features of TRN breast cancers.
Methods
Pathological and biological data were reviewed for 558 breast cancer patients treated at Kangbuk Samsung Hospital, between January 2003 and December 2009. The patients were separated into TRN breast cancer and non-TRN breast cancer groups, based on the results of immunohistochemical prognostic panels. Clinical and pathologic features were compared for the two groups. US features, including shape, orientation, margins, boundaries, echo patterns, posterior acoustic features, surrounding tissues, and microcalcifications, were determined for 41 TRN patients and 189 non-TRN controls (ER+/PR+/HER2-).
Results
Of 558 cases, 58 (10.4%) had the TRN phenotype. Four hundred and thirty-four cases (77.8%) were invasive ductal carcinomas. TRN cancer was significantly associated with specific characteristics of tumor size, nuclear grade, histologic grade, venous invasion, and lymphatic invasion. With respect to US features, TRN cancers were more likely to have an oval shape, a circumscribed margin, and marked hypoechogenicity.
Oncologists subcategorize breast cancer patients into those that are hormone-receptor positive, HER2-positive, or both hormone-receptor and HER2-negative, and treatment varies with subcategory. Triple receptor-negative (TRN) breast cancer is a subcategory that lacks expression of estrogen receptors (ER-), progesterone receptors (PR-), and human epidermal growth factor receptor 2 (HER2-) [1-4]. This subtype, which comprises 15% of all breast cancers [5], currently lacks effective targeted therapies. TRN breast cancers are typically characterized by large, high-grade tumors that have relatively high rates of recurrence and distant metastasis, and low overall survival rates [4,6-8]. TRN breast cancers are not identical to basal-like breast cancers, but have similar clinical and pathological features. Both types of breast cancer are associated with poor overall prognosis and response to chemotherapy, such as anthracycline- and taxane-based regimens [3,5,6,9,10].
Cohort studies have shown that TRN breast cancers occur at high frequencies in relatively young women and in African American women [1,2,7,11]. In Asia, TRN breast cancers occur at rates similar to those reported for Western countries [12], and are often characterized by young age of onset and dense breast tissues.
The incidence of breast cancer in Korea has been increasing over time, and is characterized by a young age of onset in comparison to Western countries [13-15]. Molecular profiling has revealed that Korean women are more likely to have the aggressive basal cell type of breast cancer than TRN breast cancer, which may account for worse prognoses of Korean women, compared to young women of European descent [15,16]. However, data specifically concerning phenotypes of TRN breast cancer in Korea are very limited. Furthermore, only a few previous reports have described the imaging features of TRN breast cancers [17,18]. The goals of the present study were to describe the TRN phenotypes of Korean breast cancer patients and to evaluate high-resolution ultrasound (HR-US) imaging features that could be used to discriminate TRN breast cancers from non-TRN breast cancers.
This retrospective study consisted of 622 consecutive patients who had surgery for breast cancer at Kangbuk Samsung Hospital between March 2003 and December 2009, 467 underwent modified radical mastectomy (MRM), 65 underwent either partial mastectomy or quadrantectomy, and 26 underwent lumpectomy (BCS) or wide excision. Of the 622 total patients, 485 underwent axillary dissection, and 73 underwent sentinel lymph node dissections. Of the total patients, 558 underwent HR-US breast examination within one month prior to surgery. Ten patients that presented with bilateral breast cancer (n = 10), and 54 who had not undergone preoperative assessment or immunohistochemical tests for ER, PR, HER2, were excluded from the image evaluations. The age range of the 588 patients was 24-88 years, and the mean age was 49.8 years. Patients who were examined from screening center were not included in this study.
All TRN and non-TRN patients were examined for the type of pathology, tumor size, nuclear grade, histologic grade, venous invasion, lymphatic invasion, lymph node metastasis, associated ductal carcinomas in situ (DCIS), distant metastasis state, and recurrence. The tumor grades of invasive carcinomas were classified as grade 1 (low), grade 2 (moderate), or grade 3 (high) based on the frequency of cell mitosis, tubule formation, and nuclear pleomorphism. DCIS cases were divided into grade 1 (low), grade 2 (moderate), or grade 3 (high) based on nuclear grade, architecture, and necrosis.
Immunohistochemistry and fluorescence in situ hybridization (FISH) performed on breast tissue specimens were used to evaluate ER, PR, and HER-2/neu status. Immunohistochemistry was performed for all breast carcinomas. Overexpression of the HER-2/neu gene (HER2+) was defined as level 3 staining intensity. Carcinomas with 0 or 1 staining intensity were considered negative for overexpression. Carcinomas with level 2 staining intensity were defined as indeterminate, and HER-2/neu overexpression was then evaluated by FISH. Based on results of the analyses, 58 of the 558 patients (10.4%) were classified as TRN, and 500 (89.6%) were classified as non-TRN.
HR-US features were analyzed for 41 TRN patients and 189 non-TRN patients, who were ER+/PR+/HER2-. In each case, one of three board-certified breast radiologists, with 5-20 years of experience in breast US, performed a preoperative HR-US examination of the breast lesion, using an HDI 5000 unit (ATL, Bothell, USA) or an IU-22 (Philips Medical Systems, Best, The Netherlands) and a 12-5 MHz linear-array transducer. Imaging data were analyzed retrospectively: images from more than two different planes per lesion were saved as a bitmap file, and a radiologist blinded to the immunophenotypes analyzed the images according to the Breast Imaging Reporting and Data System (BI-RADS) lexicon. Tumor shape (oval, round, irregular), orientation (parallel, not parallel), margins (circumscribed, indistinct, angular, microlobulated, spiculated), boundaries (abrupt interface, echogenic halo), echo patterns (hypoechoic, complex, markedly hypoechoic), posterior acoustic features (none, enhancement, shadowing), surrounding tissues (duct changes, Cooper's ligament changes or architectural distortion, skin thickening or edema), and microcalcifications were evaluated.
The chi-squared test or Fisher exact test was used to compare parameters for TRN and non-TRN patients. Student's t-test and Mann-Whitney U-test were used. The normal distribution of data in each group was confirmed with a Kolmogorov-Smirnov test before the t-test was run. A two-tailed p<0.05 indicated a significant difference between groups. Data analyses were performed with SPSS for Windows, version 16.0 (SPSS Inc., Chicago, USA).
Of the 558 breast tumors examined, there were 434 (77.8%) invasive ductal carcinomas, 72 DCIS, 16 mucinous carcinomas, 10 invasive lobular carcinomas, 7 microinvasive carcinomas, 3 papillary carcinomas, 3 medullary carcinomas, 3 mixed ductal and lobular carcinomas, 2 metaplastic carcinomas, 2 tubular carcinomas, 2 invasive cribriform carcinomas, 2 invasive micropapillary carcinomas, 1 acinic cell carcinoma, and 1 apocrine carcinoma.
The 58 patients in the TRN group had a mean age of 48 years (range, 30-82 years), and the 500 patients in the non-TRN group had a mean age of 50 years (range, 24-88 years). There was no significant difference between the two groups in the percentage of patients <40 years (Table 1). Tumors <1 cm were significantly less frequent in the TRN group than in the non-TRN group, while the opposite was true for tumors >2 cm.
Tumors with high histologic and nuclear grades were significantly more prevalent in the TRN group than in the non-TRN group (Table 1). The frequencies of venous and lymphatic invasion were also significantly higher in TRN patients than in non-TRN patients. However, the frequencies of a positive resection margin, lymph node metastasis, microcalcifications, associated DCIS, distant metastasis, and recurrence did not differ significantly between the two groups.
HR-US evaluations showed that TRN cancers were more likely to have an oval shape or circumscribed margin, were more markedly hypoechoic and less likely to have posterior shadowing than non-TRN cancers (e.g., Table 2). The other HR-US features did not differ significantly between the TRN and non-TRN groups.
Results of the present study found some pathologic features that was different between TRN from non-TRN tumors: tumor size was significantly larger, nuclear and histologic grades were significantly higher, venous and lymphatic invasion were significantly more common in the TRN group than in the non-TRN group. However, previous clinical studies found that overexpression of the HER-2/neu gene (HER2+) is associated with some of the same pathologic features, including large tumor size, axillary lymph node metastasis, negative hormone receptors, and high tumor grade [6,16]. Although these characteristics suggest poor prognoses, the limited data on treatment responses and survival of women with TRN breast cancer make it difficult to conclude that the factors do contribute to lower survival rates.
We cannot differentiate TRN breast cancers based on US imaging but it is important that the radiologist will be aware of such subtypes and that radiologist anticipate thorough preoperative examination for multifocal breast cancers, or contra-lateral breast cancer and associated axillary lymph node metastasis or distant metastasis. By comparing HR-US features in TRN and ER+/PR+/HER2-patients, the present study found imaging differences that may be useful for identifying TRN breast cancers but was not specific of the subtype. In this study we noticed that many TRN breast cancers, due to their oval shape, circumscribed margins, and low echogenicity, the TRN tumors resembled benign nodules more closely than the non-TRN tumors did (Figure 1). These features of TRN tumors may lead a radiologist to conclude that the lesion is probably benign, and have the patient wait six months before a follow-up US exam or US-guided biopsy. Furthermore, a prior study reported that ER-/PR-/HER2+ breast cancers are commonly associated with some of the same features as the TRN cancers in the present study, specifically calcifications (79%), circumscribed margins (57%), markedly low echogenicity (57%), and lack of posterior shadowing (5%), and are sometimes depicted as non-mass lesions (32%) [19]. Although, a tumor with an oval shape, a fairly well circumscribed margin, and low echogenicity could be confused with a cystic mass, results of the present study indicate that TRN breast cancer should be suspected when the tumor has those US features and is also large, palpable, and exhibits rapid growth. Biopsy should be recommended for such nodules, even if the echogenicity is not extremely low.
Results of the present study found a high incidence of microcalcifications in TRN and non-TRN groups, both of which were HER2-negative. However, Wang et al. [18] found that ER-/HER2+ breast cancers were more likely to be associated with calcifications than TRN cancers. Another previous study found that 75% of HER2+ carcinomas were associated with calcifications [16,20]. We analyzed the incidence of DCIS in the TRN and non-TRN groups, because microcalcifications previously found in TRN patients during mammography may have been psamomma bodies associated with DCIS. However, we did not find a significant association between TRN and DCIS.
The present study had several limitations. First, identification of TRN tumors was predominantly based on immunohistochemical results, and FISH analysis was only used in a limited number of cases. FISH analysis provides a more accurate determination of HER2+, due to false positives in weakly positive immunohistochemical results [21-23]. Another limitation was that the imaging analyses were limited to malignant nodules, which may have influenced interpretation by the radiologists. Third, the non-TRN group was limited to ER+/PR+/HER2-patients, because based on our experience, most young breast cancer patients are HER2-.
Although results of the present study indicate that it may be possible to differentiate TRN cancers from non-TRN cancers on the basis of specific US features, further research is needed to confirm our findings and to provide a more reliable "alarm" for cancers with very poor prognoses.
Figures and Tables
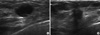 | Figure 1Ultrasonographic findings. (A) The image of a triple receptor-negative invasive ductal carcinoma in a 30-year-old-woman. It shows an oval shaped and well circumscribed markedly hypoechoic mass without posterior shadowing. (B) The image of a non-TRN (ER+/PR+/HER2-) invasive ductal carcinoma in a 47-year-old-woman. It shows irregular shaped hypoechoic mass with spiculated margin and posterior shadowing. |
References
1. Akiyama F, Iwase H. Triple negative breast cancer: clinicopathological characteristics and treatment strategies. Breast Cancer. 2009. 16:252–253.

2. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007. 109:1721–1728.

4. Sasaki Y, Tsuda H. Clinicopathological characteristics of triple-negative breast cancers. Breast Cancer. 2009. 16:254–259.

5. Elias AD. Triple-negative breast cancer: a short review. Am J Clin Oncol. 2010. 33:637–645.
6. Heitz F, Harter P, Lueck HJ, Fissler-Eckhoff A, Lorenz-Salehi F, Scheil-Bertram S, et al. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer. 2009. 45:2792–2798.

7. Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007. 13:2329–2334.

8. Tian XS, Cong MH, Zhou WH, Zhu J, Chen YZ, Liu Q. Clinicopathologic and prognostic characteristics of triple-negative breast cancer. Onkologie. 2008. 31:610–614.

9. Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007. 109:25–32.

10. Grann VR, Troxel AB, Zojwalla NJ, Jacobson JS, Hershman D, Neugut AI. Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer. 2005. 103:2241–2251.

11. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006. 295:2492–2502.

12. Tan GH, Taib NA, Choo WY, Teo SH, Yip CH. Clinical characteristics of triple-negative breast cancer: experience in an Asian developing country. Asian Pac J Cancer Prev. 2009. 10:395–398.
13. Ahn SH, Hwang UK, Kwak BS, Yoon HS, Ku BK, Kang HJ, et al. Prevalence of BRCA1 and BRCA2 mutations in Korean breast cancer patients. J Korean Med Sci. 2004. 19:269–274.

14. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea: a report from the Korean Breast Cancer Society. J Clin Oncol. 2007. 25:2360–2368.

15. Yoo KY, Kang D, Park SK, Kim SU, Kim SU, Shin A, et al. Epidemiology of breast cancer in Korea: occurrence, high-risk groups, and prevention. J Korean Med Sci. 2002. 17:1–6.

16. Seo BK, Pisano ED, Kuzimak CM, Koomen M, Pavic D, Lee Y, et al. Correlation of HER-2/neu overexpression with mammography and age distribution in primary breast carcinomas. Acad Radiol. 2006. 13:1211–1218.

17. Yang WT, Dryden M, Broglio K, Gilcrease M, Dawood S, Dempsey PJ, et al. Mammographic features of triple receptor-negative primary breast cancers in young premenopausal women. Breast Cancer Res Treat. 2008. 111:405–410.

18. Wang Y, Ikeda DM, Narasimhan B, Longacre TA, Bleicher RJ, Pal S, et al. Estrogen receptor-negative invasive breast cancer: imaging features of tumors with and without human epidermal growth factor receptor type 2 overexpression. Radiology. 2008. 246:367–375.

19. Ko ES, Lee BH, Kim HA, Noh WC, Kim MS, Lee SA. Triple-negative breast cancer: correlation between imaging and pathological findings. Eur Radiol. 2010. 20:1111–1117.

20. Karamouzis MV, Likaki-Karatza E, Ravazoula P, Badra FA, Koukouras D, Tzorakoleftherakis E, et al. Non-palpable breast carcinomas: correlation of mammographically detected malignant-appearing microcalcifications and molecular prognostic factors. Int J Cancer. 2002. 102:86–90.

21. Field AS, Chamberlain NL, Tran D, Morey AL. Suggestions for HER-2/neu testing in breast carcinoma, based on a comparison of immunohistochemistry and fluorescence in situ hybridisation. Pathology. 2001. 33:278–282.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download