Abstract
Purpose
This study is to review the initial 5-years of breast cancer management in a single hospital using the clinical data warehouse (CDW).
Methods
We reviewed the electronic medical records of 754 patients with breast cancer who were treated by a single surgeon between June 2003 and December 2007 in Seoul National University Bundang Hospital. We analyzed the epidemiological, clinical and therapeutic profiles of the breast cancer patients which were encoded and stored at the CDW.
Results
The mean age of the patients was 49.3 years and the peak incidence was in the fifth decade (36.6%). Symptomatic breast cancer was 74.6% and screening-detected breast cancer was 25.4%. Breast conserving surgery (BCS) was performed in 54.1% of all cases and the BCS rate increased annually. Immediate reconstruction after mastectomy was performed in 62 cases (17.7%). Sentinel lymph node (SLN) biopsy for nodal staging was performed in 501 cases (72.1%) and 160 cases (23.0%) underwent complete axillary lymph node dissection. The proportion of in situ and early stage invasive breast cancer was 85.0%. Six hundred and ninety three patients (92.5%) received more than one adjuvant therapy. Thirty one patients experienced local or systemic relapse after surgery and ipsilateral breast tumor recurrence (IBTR) occurred in 6 cases. The median follow-up period was 29.5 months. Two-year and 3-year disease-free survival rates were 95.9% and 94.4%.
Figures and Tables
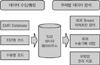 | Figure 1The process of analysis using clinical data warehouse. Clinical data was stored in a data warehouse, and clinical information was analyzed out using data mining. |
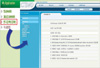 | Figure 2Items in clinical data warehouse. The various clinical information including first medical examination of patients and index of management of breast cancer were obtained through the clinical data warehouse. |
 | Figure 3Trends of operation methods. Breast conserving surgery (BCS) was performed 54.1% of all cases and the BCS rate increased annually. |
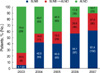 | Figure 4Trends of operation for axillary staging. Sentinel lymph node biopsy increased annually and was performed in 192 cases (89.3%) in 2007. SLNB=sentinel lymph node biopsy; ALND=axillary lymph node dissection. |
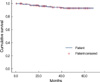 | Figure 5Overall disease-free survival. During 29.5 months of median follow-up, the 3-yr disease-free survival rate was 94.4%. |
 | Figure 6Analysis of immediate reconstruction rate using clinical data warehouse. The result of immediate reconstruction rate after mastectomy was reported easily by setting the condition for the period. |
References
1. Ministry of Health and Welfare. Korea Central Cancer Registry. 2002 Annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. 2003. Seoul:
2. Ahn SH, Yoo KY. Chronological changes of clinical characteristics in 31,115 new breast cancer patients among Koreans during 1996-2004. Breast Cancer Res Treat. 2006. 99:209–214.

3. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002. 347:1227–1232.

4. Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998. 352:930–942.
5. Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005. 365:1687–1717.
6. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005. 353:1673–1684.

7. Madigan MP, Ziegler RG, Benichou J, Byrne C, Hoover RN. Proportion of breast cancer cases in the United States explained by well-established risk factors. J Natl Cancer Inst. 1995. 87:1681–1685.

8. Go BJ, Kim MH, Chang SH, Paik IW. A clinical review of breast cancer. J Korean Surg Soc. 1998. 55:959–972.
9. Son BH, Kwak BS, Kim JK, Kim HJ, Hong SJ, Lee JS, et al. Changing patterns in the clinical characteristics of Korean patients with breast cancer during the last 15 years. Arch Surg. 2006. 141:155–160.

10. Lee JS, Bae YT. Clinical analysis of breast cancer patients treated with surgery. J Korean Breast Cancer Soc. 2004. 7:174–179.

11. Godfrey PM, Godfrey NV, Romita MC. Immediate autogenous breast reconstruction in clinically advanced disease. Plast Reconstr Surg. 1995. 95:1039–1044.

12. Styblo TM, Lewis MM, Carlson GW, Murray DR, Wood WC, Lawson D, et al. Immediate breast reconstruction for stage III breast cancer using transverse rectus abdominis musculocutaneous (TRAM) flap. Ann Surg Oncol. 1996. 3:375–380.

13. Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997. 349:1864–1867.

14. Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, et al. The sentinel node in breast cancer: a multicenter validation study. N Engl J Med. 1998. 339:941–946.

15. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001. 30:96–102.

16. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008. 26:778–785.

17. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002. 347:1233–1241.

18. Tan-Chiu E, Yothers G, Romond E, Geyer CE Jr, Ewer M, Keefe D, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005. 23:7811–7819.

19. Health Insurance Review Agency. Report on the realities of information delivery from medical care providers. 2005. Seoul:




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download