Abstract
Purpose
The objective of this study was to evaluate the change in the practice patterns for managing hereditary breast and ovarian cancer (HBOC) among Korean physicians after the Korean Hereditary Breast Cancer (KOHBRA) study.
Methods
The first survey was performed from July to August 2007, at the initiation of the KOHBRA study, and the follow-up survey was conducted from July to December 2009. Members of the Korean Breast Cancer Society were invited to participate in the study by e-mail. The 2009 survey was conducted with a self-administered questionnaire concerning HBOC management and was identical to the previous questionnaire.
Results
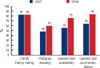
According to the 2009 survey, most physicians (60.0%) tended to draw a pedigree (48.0% in 2007 survey). The rate of genetic test recommendations for patients at risk for HBOC was higher in the 2009 survey (84.0%) than that in the 2007 survey (64.0%). Physicians tended to select a BRCA genetic testing candidate more appropriately than in the previous survey (42.4% answered right in 2007 survey; 74.4% in 2009 survey). Fifteen of 25 participants (60.0%) provided genetic counseling before their patients underwent a genetic test, which was higher than that (40.0%) in the 2007 survey. According to the 2009 survey, half of the genetic counseling was being conducted by KOHBRA study research nurses; whereas most of the genetic counseling was conducted by physicians in 2007.
Figures and Tables
References
1. Ko SS. Chronological changing patterns of clinical characteristics of Korean breast cancer patients during 10 years (1996-2006) using nationwide breast cancer registration on-line program: biannual update. J Surg Oncol. 2008. 98:318–323.

2. Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996. 77:2318–2324.

3. Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van't Veer L, Garber JE, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002. 346:1616–1622.

4. Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998. 90:1371–1388.

5. Hartmann LC, Schaid DJ, Woods JE, Crotty TP, Myers JL, Arnold PG, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999. 340:77–84.

6. Oh JH, Noh DY, Choe KJ, Kang SB, Kim LS, Ro MS, et al. Germline mutation of BRCA1 gene in Korean breast and ovarian cancer patients. J Korean Cancer Assoc. 1995. 27:1061–1069.
7. Kang HC, Kim IJ, Park JH, Kwon HJ, Won YJ, Heo SC, et al. Germline mutations of BRCA1 and BRCA2 in Korean breast and/or ovarian cancer families. Hum Mutat. 2002. 20:235.

8. Choi DH, Lee MH, Bale AE, Carter D, Haffty BG. Incidence of BRCA1 and BRCA2 mutations in young Korean breast cancer patients. J Clin Oncol. 2004. 22:1638–1645.

9. Kim EK, Kim KS, Park SK, Ahn SH, Lee MH, Kim SW, et al. The Korean Hereditary Breast Cancer (KOHBRA) study: protocol rewiew. J Breast Cancer. 2007. 10:241–247.

10. Kim KS, Kim SW, Lee MH, Ahn SH, Park SK. Korean Breast Cancer Society. Practice patterns of surgeons for the management of hereditary breast cancer in Korea. J Breast Cancer. 2008. 11:95–101.

11. Friedman LS, Ostermeyer EA, Szabo CI, Dowd P, Lynch ED, Rowell SE, et al. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994. 8:399–404.

12. Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995. 378:789–792.

13. Shattuck-Eidens D, Oliphant A, McClure M, McBride C, Gupte J, Rubano T, et al. BRCA1 sequence analysis in women at high risk for susceptibility mutations. Risk factor analysis and implications for genetic testing. JAMA. 1997. 278:1242–1250.

14. Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998. 62:145–158.

15. Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003. 72:1117–1130.

16. Chenevix-Trench G, Milne RL, Antoniou AC, Couch FJ, Easton DF, Goldgar DE. An international initiative to identify genetic modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: the Consortium of Investigators of Modifiers of BRCA1 and BRCA2 (CIMBA). Breast Cancer Res. 2007. 9:104.

17. Nelson HD, Huffman LH, Fu R, Harris EL. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005. 143:362–379.

18. Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998. 62:676–689.

19. Ahn SH, Hwang UK, Kwak BS, Yoon HS, Ku BK, Kang HJ, et al. Prevalence of BRCA1 and BRCA2 mutations in Korean breast cancer patients. J Korean Med Sci. 2004. 19:269–274.

20. Malone KE, Daling JR, Neal C, Suter NM, O'Brien C, Cushing-Haugen K, et al. Frequency of BRCA1/BRCA2 mutations in a population-based sample of young breast carcinoma cases. Cancer. 2000. 88:1393–1402.

21. Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010. 28:893–901.

22. American Society of Clinical Oncology. Resource document for curriculum development in cancer genetics education. J Clin Oncol. 1997. 15:2157–2169.
23. Green MJ, Peterson SK, Baker MW, Harper GR, Friedman LC, Rubinstein WS, et al. Effect of a computer-based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: a randomized controlled trial. JAMA. 2004. 292:442–452.

24. Bioethics and Biosafety Act. Korea Ministry of Government Legislation. Accessed July 27th, 2010. http://www.law.go.kr/LSW/LsInfoP.do?lsiSeq=87356#0000.
25. H.R.493: The Genetic Information Nondiscrimination Act of 2008. U.S. Government Printing Office. Accessed July 27th, 2010. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=110_cong_bills&docid=f:h493enr.txt.pdf.
26. The NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast and Ovarian - v.1.2010. National Comprehensive Cancer Network. Accessed July 28th, 2010. http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf.
27. Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005. 97:1652–1662.

28. Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van't Veer L, Garber JE, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004. 22:1055–1062.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download