Abstract
Purpose
To analyze the effect of the growth control on human breast cancer cells with genistein treatment and to investigate the mechanism of genistein-induced G2/M arrest in T47D and MDA-MB231 breast carcinoma cells by Cdc25C expression.
Methods
We analysed the proliferartion of the two cell lines by using MTT proliferation assay, flow cytometric analysis, real-time quantitative RT-PCR and western blotting and investigated the effect of genistein on cell survival, cellular toxicity, cell cycle progression-related genes and their mRNA and protein alterations.
Results
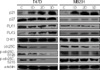
The DNA flow cytometric analysis of both cell lines treated with genistein showed a dose-dependent growth inhibition and accumulation in the G2/M phase of cell cycle. The expression of p21 mRNA and protein increased in both cell lines following genistein treatment but p27 expression was unchanged. Furthermore, decreased Cdc25C expression with decreased polo-like kinase (PLK) 1 expression and increased PLK3 expression were observed after genistein treatment. The decreased level of Cdc25C in the nucleus was associated with decreased phosphorylation of Cdc25C by PLK1. The expression of PLK3 was increased with a dose-dependent and a time-dependent manner and was associated with decreased Cdc25C expression. Check point kinase (CHK) 1 and CHK2 revealed different expression patterns each other. The CHK1 expression was independent of the presence of genestein. CHK2 expression increased in MDA-MB231 cells associated with decreased Cdc25C expression but not in T47D.
Conclusion
These results suggest that genistein induces a G2/M arrest in human breast cancer cells, the mechanism of which is due, in part, to decreased in Cdc25C phosphatase by a regulatory effect of PLK1, PLK3, and CHK2 as well as increased expression of the cyclin dependent kinase inhibitor p21(WAF1/CIP1).
Figures and Tables
 | Figure 1Concentration dependent growth inhibition of estrogen receptor (ER)-positive (T47D) and ER-negative (MDA-MB231) breast cancer cells by genistein. Twenty-four hours after inoculation, cells were cultured for 3 days in the absence or presence of genistein at concentratins ranging from 0.1 to 100 µM. The data represent the mean±SD of six independent experiments. |
 | Figure 2Changes of cell densitiy in T47D cells (A, C) and MDA-MB231cells (B, D). Each cells were treated with 0.1% DMSO (control) or 100 µM of genistein for 3 days. Genistein-treated cells are decreased in cellularity than control. |
 | Figure 3DNA-fluorescence histogram of T47D and MDA-MB231 cells after genistein treatment. T47D (A) and MDA-MB231 (B) cells were treated with 0.1% DMSO (CON) or 100 µM of genistein (GEN) for 72 hr. Three days after genistein treatment, there was 3.1-fold increase in the percent of T47D cells in G2/M and 4.2-fold increase in the percent of MDA-MB231cells in G2/M, respectively. |
 | Figure 4Real time quantitative analysis for p21 mRNA of genistein-treated T47D and MDA-MB231 cells. Each cell (5×105 cells) was treated with 0-100 µM genistein for 3 days or 100 µM of genistein for 0.5, 1, 2, 3 days. |
 | Figure 5Real time quantitative analysis for p27 mRNA of genistein-treated T47D and MDA-MB231 cells. Each cell (5×105 cells) treated with 0-100 µM genistein for 3 days or 100 µM of genistein for 0.5, 1, 2, 3 days. |
 | Figure 6Real time quantitative analysis for PLK1 mRNA and CHK1 mRNA of genistein-treated T47D and MDA-MB231 cells. Each cell (5×105 cells) treated with 100 µM of genistein for 0.5, 1, 2, 3 days. |
 | Figure 7Real time quantitative analysis for PLK3 of genistein-treated T47D and MDA-MB231 cells. Each cell (5×105 cells) treated with 0-100 µM genistein for 3 days or 100 µM of genistein for 0.5, 1, 2, 3 days. |
 | Figure 8Real time quantitative analysis for CHK2 mRNA of genistein-treated T47D and MDA-MB231 cells. Each cell (5×105 cells) treated with 0-100 µM genistein for 3 days or 100 µM of genistein for 0.5, 1, 2, 3 days. |
 | Figure 9Real time quantitative analysis for Cdc25C mRNA of genistein-treated T47D and MDA-MB231 cells. Each cell (5×105 cells) treated with 0-100 µM genistein for 3 days or 100 µM of genistein for 0.5, 1, 2, 3 days. |
References
1. Cappelletti V, Fioravanti L, Miodini P, Di Fronzo G. Genistein blocks breast cancer cells in the G(2)M phase of the cell cycle. J Cell Biochem. 2000. 79:594–600.

2. Casagrande F, Darbon JM. p21CIP1 is dispensable for the G2 arrest caused by genistein in human melanoma cells. Exp Cell Res. 2000. 258:101–108.

3. Chang KL, Kung ML, Chow NH, Su SJ. Genistein arrests hepatoma cells at G2/M phase: involvement of ATM activation and upregulation of p21waf1/cip1 and Wee1. Biochem Pharmacol. 2004. 67:717–726.

4. Choi YH, Zhang L, Lee WH, Park KY. Genistein-induced G2/M arrest is associated with the inhibition of cyclin B1 and the induction of p21 in human breast carcinoma cells. Int J Oncol. 1998. 13:391–396.

5. Ding H, Duan W, Zhu WG, Ju R, Srinivasan K, Otterson GA, et al. P21 response to DNA damage induced by genistein and etoposide in human lung cancer cells. Biochem Biophys Res Commun. 2003. 305:950–956.

6. Frey RS, Li J, Singletary KW. Effects of genistein on cell proliferation and cell cycle arrest in nonneoplastic human mammary epithelial cells: involvement of Cdc2, p21(waf/cip1), p27(kip1), and Cdc25C expression. Biochem Pharmacol. 2001. 61:979–989.

7. Frey RS, Singletary KW. Genistein activates p38 mitogen-activated protein kinase, inactivates ERK1/ERK2 and decreases Cdc25C expression in immortalized human mammary epithelial cells. J Nutr. 2003. 133:226–231.

8. Kuzumaki T, Kobayashi T, Ishikawa K. Genistein induces p21(Cip1/WAF1) expression and blocks the G1 to S phase transition in mouse fibroblast and melanoma cells. Biochem Biophys Res Commun. 1998. 251:291–295.

9. Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987. 262:5592–5595.

11. Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, et al. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989. 49:5111–5117.
12. Linassier C, Pierre M, Le Pecq JB, Pierre J. Mechanisms of action in NIH-3T3 cells of genistein, an inhibitor of EGF receptor tyrosine kinase activity. Biochem Pharmacol. 1990. 39:187–193.

13. Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, Montesano R, et al. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci U S A. 1993. 90:2690–2694.

14. Mueller SC, Yeh Y, Chen WT. Tyrosine phosphorylation of membrane proteins mediates cellular invasion by transformed cells. J Cell Biol. 1992. 119:1309–1325.

15. Shao ZM, Alpaugh ML, Fontana JA, Barsky SH. Genistein inhibits proliferation similarly in estrogen receptor-positive and negative human breast carcinoma cell lines characterized by P21WAF1/CIP1 induction, G2/M arrest, and apoptosis. J Cell Biochem. 1998. 69:44–54.

16. Upadhyay S, Neburi M, Chinni SR, Alhasan S, Miller F, Sarkar FH. Differential sensitivity of normal and malignant breast epithelial cells to genistein is partly mediated by p21(WAF1). Clin Cancer Res. 2001. 7:1782–1789.
17. Bahassi el M, Myer DL, McKenney RJ, Hennigan RF, Stambrook PJ. Priming phosphorylation of Chk2 by polo-like kinase 3 (Plk3) mediates its full activation by ATM and a downstream checkpoint in response to DNA damage. Mutat Res. 2006. 596:166–176.

18. Boutros R, Dozier C, Ducommun B. The when and wheres of CDC25 phosphatases. Curr Opin Cell Biol. 2006. 18:185–191.

20. Jiang N, Wang X, Jhanwar-Uniyal M, Darzynkiewicz Z, Dai W. Polo box domain of Plk3 functions as a centrosome localization signal, overexpression of which causes mitotic arrest, cytokinesis defects, and apoptosis. J Biol Chem. 2006. 281:10577–10582.

22. van de Weerdt BC, Medema RH. Polo-like kinases: a team in control of the division. Cell Cycle. 2006. 5:853–864.

23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001. 25:402–408.

24. Yoon DS, Song KH, Kim ST. Different expression of p27(kip1) and p57(kip2) by genistein treatment in MDA-MB-231 human breast cancer cells. J Korean Breast Cancer Soc. 2004. 7:98–103.

25. Agarwal R. Cell signaling and regulators of cell cycle as molecular targets for prostate cancer prevention by dietary agents. Biochem Pharmacol. 2000. 60:1051–1059.

26. Waite KA, Sinden MR, Eng C. Phytoestrogen exposure elevates PTEN levels. Hum Mol Genet. 2005. 14:1457–1463.

27. Masuda Y, Nishida A, Hori K, Hirabayashi T, Kajimoto S, Nakajo S, et al. Beta-hydroxyisovalerylshikonin induces apoptosis in human leukemia cells by inhibiting the activity of a polo-like kinase 1 (PLK1). Oncogene. 2003. 22:1012–1023.

28. Zhu H, Chang BD, Uchiumi T, Roninson IB. Identification of promoter elements responsible for transcriptional inhibition of polo-like kinase 1 and topoisomerase IIalpha genes by p21(WAF1/CIP1/SDI1). Cell Cycle. 2002. 1:59–66.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download