Abstract
Purpose
Extended treatment with aromatase inhibitors (AIs) after tamoxifen has shown effectiveness in postmenopausal patients with breast cancer. However it is very difficult to start on AIs for patients who become postmenopausal after tamoxifen because tamoxifen is a selective estrogen receptor modulator (SERM) that influences menopause, confusing the menopausal status of patients. We assessed the menopausal status and hormone concentrations at the start of letrozole treatment in women with breast cancer who were premenopausal when diagnosed with breast cancer and who became postmenopausal during 5 years of tamoxifen therapy.
Methods
We evaluated 164 patients with breast cancer who received extended letrozole therapy between May 2006 and December 2007. All had been premenopausal at diagnosis but became postmenopausal during 5 years of tamoxifen therapy. Menopause was defined as amenorrhea for >1 year, serum follicle stimulating hormone (FSH) concentration ≥30 mIU/mL or serum estradiol (E2) concentrations ≤20 pg/mL. FSH and E2 concentrations were monitored for 2 years after starting letrozole therapy.
Results
The median ages of the 164 patients were 45 years at surgery, 46 years when they became amenorrheic, and 50 years at the start of letrozole treatment. Of the 164 patients, 157 (95.7%) were amenorrheic, 14 (9.3%) had FSH concentrations ≥30 mIU/mL and 113 (70.2%) had E2 concentrations ≤20 pg/mL at the start of letrozole. FSH concentrations ≥30 mIU/mL were observed in 87 patients (57.6%) after 6 months of letrozole and in 133 (88.1%) after 2 years, and E2 concentrations ≤20 pg/mL were observed in 164 patients (100%) after 2 years. Times to reach FSH ≥30 mIU/mL and E2 levels ≤20 pg/mL were not significantly related to age at surgery (p=0.836 and p=0.228, respectively), at start of letrozole (p=0.855 and p=0.357, respectively), or at amenorrhea (p=0.098 and p=0.154, respectively).
Conclusion
Applying postmenopausal ranges of hormone concentrations observed in normal healthy people to patients who completed 5 years of tamoxifen is inappropriate, because tamoxifen itself may affect FSH concentration. Further studies should focus on identifying an indicator of ovarian function so that these patients can start extended hormone therapy.
Since estrogen acts as a promoter of breast carcinogenesis,(1) many anti-estrogen therapies have been utilized in breast cancer patients, including the use of selective estrogen receptor modulators and ovulatory ablation. Tamoxifen, an anti-estrogen agent, has been used for more than 20 yr, and has become the most popular endocrine agent used in women with breast cancer.(2,3) Recently, extended treatment with third-generation aromatase inhibitors (AIs) has been shown effective in postmenopausal women with breast cancer.(4) The National Comprehensive Cancer Network (NCCN) recommends adjuvant endocrine therapy for postmenopausal women with breast cancer who are under age 60 yr and who have completed 5 yr of tamoxifen treatment. However, to start AIs, women should have postmenopausal levels of follicle stimulating hormone (FSH) and estradiol (E2), since AIs are not effective in women with continued ovarian function.
The age distribution of patients with breast cancer in Korea differs markedly from that in Western countries. In Korea, about 60% of newly diagnosed patients are premenopausal, compared with approximately one-third in Western countries.(5-7) Prognosis is poorer in younger than in older patients, raising questions about the optimal treatment for these younger, mostly premenopausal patients. Treatment of postmenopausal breast cancer patients with letrozole after 5 yr of tamoxifen has been shown to prolong disease-free and overall survival.(4) However, not all amenorrheic patients can start letrozole immediately after tamoxifen therapy since they may not be postmenopausal. That is, despite being amenorrheic, these patients must wait to start letrozole until their reproductive hormones reach postmenopausal concentrations according to NCCN guidelines. Tamoxifen, however, is a selective estrogen receptor modulator (SERM), thereby influencing hormone concentrations. To determine if the definition of menopause which the NCCN guideline suggesting is sufficient to determine ovarian function, we evaluated the menopausal state and hormone concentrations of patients with breast cancer who were premenopausal at diagnosis, had just finished 5 yr of tamoxifen therapy, had become postmenopausal during tamoxifen therapy, and had started letrozole therapy.
Between May 2006 and December 2007, we enrolled 170 patients with breast cancer, all of whom had started extended letrozole therapy (2.5 mg once daily) at the Asan Medical Center. All patients were premenopausal at diagnosis of breast cancer and became postmenopausal after completing 5 yr of tamoxifen therapy (10 mg twice daily). At that time the Asan Medical Center defined postmenopausal status as amenorrhea for at least 1 year or serum FSH concentration ≥30 mIU/mL or E2 concentration ≤20 pg/mL.(5) Thus, patients who had completed 5 yr of tamoxifen therapy, who met this definition of postmenopausal status, and who agreed to extended treatment with letrozole were enrolled.
Age at menopause was recorded following a telephone interview with each patient. Hormone concentrations, age at the time of surgery, and age when starting letrozole were collected retrospectively from patients' medical records. Concentrations of FSH and E2 were measured at the start of letrozole treatment and every 6 months thereafter for 2 yr.
We assessed the correlations between times to FSH ≥30 mIU/mL and E3 ≤20 pg/mL with patient ages at surgery, starting letrozole, and becoming menopausal, as well as with duration of amenorrhea before starting letrozole and treatment modality. Statistical significance levels among the different age groups were tested by one-way analysis of variance. All statistical calculations were performed using SPSS software version 12.0K (SPSS Inc., Chicago, USA), with p-values <0.05 considered statistically significant.
Of the 170 enrolled patients, six were excluded due to missing clinical data, leaving 164 patients in the study. Median patient age was 45 yr at the time of surgery, 46 yr at menopause, and 50 yr at the start of letrozole treatment. Nearly 50% of the patients had more than 4 yr of amenorrhea before starting letrozole (Table 1). The clinical profiles of the patients are shown in Table 2.
Among the 164 patients, 157 were amenorrheic (95.7%), 14 (9.3%) had FSH concentrations ≥30 mIU/mL and 113 (70.2%) had E2 concentrations ≤20 pg/mL at the start of letrozole treatment (Figure 1). FSH concentrations ≥30 mIU/mL were observed in 87 of 151 patients (57.6%) after 6 months of letrozole and in 133 of 151 (88.1%) after 2 yr. At 2 yr, 18 patients (11.9%) still had low (<30 mIU/mL) serum FSH concentrations (Table 3, Figure 2). The mean ±SD and median times to reach FSH concentrations ≥30 mIU/mL were 7.6±5.3 months and 6 months, respectively. Mean time to reach FSH ≥30 mIU/ mL was significantly shorter in patients who did than did not receive chemotherapy (7.6 vs. 18.4 months, p< 0.001).
E2 concentrations ≤20 pg/mL, observed in 113 patients (70.2%) at the start of letrozole therapy, were observed in all 164 patients (100%) after 2 yr (Table 4, Figure 3). The mean±SD time to reach an E2 concentration ≤20 pg/mL was 2.6±4.6 months.
The mean times to FSH ≥30 mIU/mL and E2 ≤20 pg/ mL were not significantly related to patient age at surgery (p=0.836 and p=0.228, respectively), age at the start of letrozole (p=0.855 and p=0.357, respectively), age at menopause (p=0.980 and p=0.154, respectively), or duration of amenorrhea before the start of letrozole (p=0.691 and p=0.551, respectively) (Table 5).
Patients who were younger at diagnosis had a significantly longer duration of amenorrhea than patients who were older at diagnosis (p=0.019) (Table 6).
Among the 164 patients, four restarted vaginal bleeding and were diagnosed as having resumed menstruation; these patients stopped letrozole therapy.
The average age at diagnosis of breast cancer is lower in Asian than in Western countries.(5,6,8) Moreover, increased use of screening tests has increased the proportion of younger patients diagnosed with the disease,(8) leading to increased interest in treatment optimization for young, generally premenopausal women. AIs have shown effectiveness in postmenopausal women with breast cancer as up-front therapy.(9,10) Younger, premenopausal patients who become postmenopausal during treatment with an AI may be considered for a switch(11,12) or extended therapy.(13,14) Although menopause in generally healthy women is usually determined by age and clinical menstruation status, hormonal concentrations (e.g., FSH, luteinizing hormone [LH] and E2) are included when evaluating menopause in younger patients and those who received chemotherapy and hormonal therapy.(15) Patients with breast cancer experience early menopause due to ovarian suppression induced by chemotherapy or antiestrogen therapy.(7,16) We found that the median period of amenorrhea prior to the start of letrozole treatment was 4 yr, with 94.9% of our patients being amenorrheic for more than 1 yr before starting letrozole. In contrast, only 9.3% of our patients had serum FSH concentrations ≥30 mIU/mL at the start of letrozole treatment, indicating that FSH concentration did not sufficiently reflect the exact menopausal state of these patients. We also found that 70.2% of our patients had serum E2 concentrations ≤20 pg/mL when starting letrozole, with 100% of our patients having low E2 after 2 yr of letrozole treatment, suggesting that E2 concentration may better correspond to amenorrhea before the start of AI treatment than FSH concentrations.
Since tamoxifen has estrogenic effects(17,18) and affects the hypothalamus-pituitary axis,(19,20) a combination of chemotherapy and tamoxifen reduces serum concentrations of LH and FSH in both pre- and postmenopausal women with breast cancer. These effects are observed throughout treatment with tamoxifen.(19) In premenopausal women, the combination of chemotherapy and tamoxifen increases the output of ovarian estrogen. Ovarian estrogen secretion continues longer in patients treated with tamoxifen and chemotherapy than in those treated with chemotherapy alone, with the former showing feedback inhibition of gonadotropin secretion. Eventually, however, the ovaries fail. Tamoxifen has estrogen-like activity on gonadotropin secretion in postmenopausal women.(21,22) In premenopausal women, there are incomplete increases in LH and FSH levels once chemotherapy destroys the ovary, with gonadotropin concentrations remaining constant until tamoxifen therapy is stopped. Once tamoxifen is stopped gonadotropins concentrations increase to the postmenopausal range.(19,23) This may explain, at least in part, why the FSH concentrations in our patients did not increase to ≥30 mIU/mL despite these women being postmenopausal. These findings suggest that FSH concentrations cannot be used to determine ovarian function in patients with breast cancer.
In postmenopausal patients with early-stage breast cancer, the endocrine effects of tamoxifen have been compared with those of adjuvant letrozole. Although both drugs decreased E2 concentrations, the decrease was greater in the letrozole group. Moreover, FSH concentrations decreased in patients treated with tamoxifen, but increased slightly in patients treated with letrozole.(24)
Determining menopausal status is important prior to AI treatment in patients with breast cancer. AI treatment is indicated for postmenopausal patients with breast cancer but is contraindicated for women with functioning ovaries.(25) In most women, amenorrhea after chemotherapy is permanent(26,27) although menstruation resumes in some patients.(7,16,28) Indeed, we found that 4 of our 164 patients (2.4%) recovered menstruation after letrozole therapy, with one beginning to menstruate after 3 months, and 2 after 17 months each, of letrozole administration, resulting in a discontinuation of letrozole in these women. The NCCN guidelines do not include an exact method for determining ovarian function in women who become amenorrheic after chemotherapy and who receive antiestrogen therapy. Definitions of menopause have varied among clinical studies and countries.(15) If menopause is defined as age >50 yr and FSH concentration ≥30 mIU/mL, only 9.3% of our patients could have started extended letrozole therapy after completing 5 yr of tamoxifen. Nevertheless, except for the four patients who resumed menstruating, all of our patients were started on letrozole therapy despite their FSH concentrations being low. After 1 and 2 yr, 57.6% and 88.1% of our patients, respectively, reached FSH concentrations ≥30 mIU/mL. The FSH level is low at the beginning of letrozole because of a tamoxifen effect and after stared letrozole FSH concentrations increased. Letrozole itself may facilitate a increase in FSH concentrations.(24)
Late clinical recovery of ovarian function has been observed among women with treatment-induced amenorrhea, thus rendering AI therapy ineffective.(29) In these women, a combination of letrozole and ovary suppression therapy may be effective. Extended letrozole therapy has also shown improved outcomes in patients with early breast cancer.(30) To date, in the absence of accurate determinations of menopause, no guidelines have formulated an exact time and duration for extended AI treatment. Thus, due to unclear ovarian function, physicians must decide whether to delay AI treatment until FSH is ≥30 mIU/m or to continue extended endocrine therapy immediately after finishing tamoxifen therapy. Since only four of our 164 patients restarted menstruation, three after 18 months of extended letrozole therapy, hormone concentrations should be monitored periodically in patients who start letrozole therapy. Although letrozole is started earlier for amenorrheic patients with low FSH, regular measurements of serum concentrations of reproductive hormones, together with a physical examination, may prevent the side effects of treatment and determine whether ovarian function has resumed.
This study had several limitations, including its single-center retrospective design and the lack of a control group. Comparing changes in hormone concentrations between patients who did and did not receive extended letrozole therapy could have more meaningful results. Our definition of postmenopause differed from that in NCCN guidelines. We therefore included amenorrheic patients, although their FSH concentrations were not in a postmenopausal range. These patients may have been perimenopausal when they started letrozole, which is not currently recommended. However we continuously checked their hormonal levels and regular outpatient treatment to avoid side effect and to recognize resume of menstruation and ovary function. Furthermore, this is the first study to objectively evaluate menopause and hormone status changes among Korean women with breast cancer who were treated with letrozole after tamoxifen. Although it is very important to determine ovarian function in candidates for AI treatment, it is difficult to exactly determine ovarian function in women who have received chemotherapy and endocrine therapy. Thus, the results of future, prospective, multicenter studies on menstrual state and hormonal changes in patients with breast cancer who received tamoxifen therapy may play an important role in patient outcomes.
Determining exact menopausal status is important in treating patients with breast cancer. Patients with continued ovarian function should not start AIs, but AI treatment should not be delayed in patients who become postmenopausal during tamoxifen treatment because AIs can prolong their survival. According to current guidelines, however, amenorrhea for more than 1 yr and postmenopausal ranges of FSH and E2 concentrations can delay treatment for patients who have completed 5 yr of tamoxifen treatment. Hormone level can be in normal range because the patients still have functioning ovaries, in some patients, hormone level do not reach postmenopausal range because of tamoxifen effect although they do not have functioning ovaries.
Applying postmenopausal hormonal ranges of normal healthy people is inappropriate in determining the postmenopausal status of breast cancer patients who have completed 5 years of tamoxifen therapy, because tamoxifen itself changes FSH concentrations. Additional studies are required to identify indicators of ovarian function in these women so that they can be started on proper endocrine therapy.
Figures and Tables
 | Figure 1Proportion of the patients for each elements of postmenopausal definition, upon start of letrozole therapy. Among the 164 patients, 157 were amenorrheic (95.7%), 14 (9.3%) had FSH concentrations ≥30 mIU/mL and 113 (70.2%) had E2 concentrations ≤20 pg/mL at the start of letrozole treatment, only 10 patients satisfied all 3 elements of postmenopausal defifnition upon start of letrozole therapy.
FSH=follicle stimulating hormone; E2=estradiol.
|
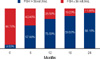 | Figure 2Proportion of patients with FSH concentrations ≥30 mIU/mL, assessed at 6-month intervals FSH concentrations ≥30 mIU/mL were observed in 14 of 151 patients (9.3%) at the start of letrozole, 87 of 151 patients (57.6%) after 6 month of letrozole and in 133 of 151 (88.1%) after 2 yr. At 2 yr, 18 patients (11.9%) still had low (<30 mIU/mL) serum FSH concentrations.
FSH=follicle stimulating hormone.
|
 | Figure 3Proportions of the patients with E2 concentration <20 pg/mL, assessed at 6-month intervals E2 concentrations ≤20 pg/ mL, observed in 113 patients (70.2%) at the start of letrozole therapy, 145 patietns (90.1%) after 6 month of letrozole and in all 164 patients (100%) after 2 yr.
E2=estradiol.
|
References
1. Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, Banerjee S, Koenig KL, Shore RE, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995. 87:190–197.

2. Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res. 1990. 50:4177–4189.
3. Jordan VC. Long-term adjuvant tamoxifen therapy for breast cancer. Breast Cancer Res Treat. 1990. 15:125–136.

4. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003. 349:1793–1802.

5. The Korean. Nationwide Korean breast cancer data of 2004 using breast cancer registration program. J Breast Cancer. 2006. 9:151–161.

6. Ahn SH, Yoo KY. Korean Breast Cancer Society. Chronological changes of clinical characteristics in 31,115 new breast cancer patients among Koreans during 1996-2004. Breast Cancer Res Treat. 2006. 99:209–214.

7. Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006. 24:5769–5779.

8. Morimoto T, Okazaki M, Endo T. Current status and goals of mammographic screening for breast cancer in Japan. Breast Cancer. 2004. 11:73–81.

9. Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for a adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomized trial. Lancet. 2002. 359:2131–2139.
10. Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, et al. Five years of letrozole compared with tamoxifen as initial a adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007. 25:486–492.

11. Jonat W, Gnant M, Boccardo F, Kaufmann M, Rubagotti A, Zuna I, et al. Effectiveness of switching from adjuvant tamoxifen to anastrozole in postmenopausal women with hormone-sensitive early-stage breast cancer: a meta analysis. Lancet Oncol. 2006. 7:991–996.

12. Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004. 350:1081–1092.

13. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005. 97:1262–1271.

14. DeGrendele H. Benefit of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. Clin Breast Cancer. 2003. 4:311–312.

15. Aksoy S, Dizdar O, Altundag K. Definition of postmenopausal status, age of the breast cancer patients and the outcome of aromatase inhibitors treatment. Breast. 2008. 17:433–435.

16. Kim HA, Shin DS, Moon NM, Paik NS, Noh WC. The incidence of chemotherapy-induced amenorrhea and recovery in young (45-year-old) breast cancer patients. J Breast Cancer. 2009. 12:20–26.

17. Kavak ZN, Binöz S, Ceyhan N, Pekin S. The effect of tamoxifen on the endometrium, serum lipids and hypothalamus pituitary axis in the postmenopausal breast cancer patients. Acta Obstet Gynecol Scand. 2000. 79:604–607.

18. Ellmén J, Hakulinen P, Partanen A, Hayes DF. Estrogenic effect of toremifen and tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res Treat. 2003. 82:103–111.

19. Jordan VC, Fritz NF, Tormey DC. Endocrine effects of adjuvant chemotherapy and long-term tamoxifen administration on node-positive patients with breast cancer. Cancer Res. 1987. 47:624–630.
20. Samaan NA, deAsis DN Jr, Buzdar AU, Blumenschein GR. Pituitary-ovarian function in breast cancer patients on a adjuvant chemoimmunotherapy. Cancer. 1978. 41:2084–2087.

21. McFadyen IJ, Raab G, Forrest AP, Langlands AO, Stewart HJ, Roberts MM, et al. The effect of tamoxifen and stilboestrol on plasma hormone levels in postmenopausal women with advanced breast cancer. Clin Oncol. 1979. 5:251–256.
22. Golder MP, Phillips ME, Fahmy DR, Preece PE, Jones V, Henk JM, et al. Plasma hormones in patients with advanced breast cancer treated with tamoxifen. Eur J Cancer. 1976. 12:719–723.

23. Delrio G, Placido S, pagliarulo C, d'Istria M, Fasano S, Marinelli A, et al. Hypothalamic-pituitary-ovarian axis in women with operable breast cancer treated with adjuvant CMF and tamoxifen. Tumori. 1986. 72:53–61.

24. Rossi E, Morabito A, Di Rella F, Esposito G, Gravina A, Labonia V, et al. Endocrine effects of adjuvant letrozole compared with tamoxifen in hormone-responsive postmenopausal patients with early breast cancer: the HOBOE trial. J Clin Oncol. 2009. 27:3192–3197.

25. Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, et al. American Society of Clinical Oncology practice giudeline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010. 28:3784–3796.

26. Bines J, Oleske DM, Cobleigh MA. Ovarian fuction in premenopusal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996. 14:1718–1729.

27. Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999. 17:2365–2370.

28. Amir E, Seruga B, Freedman O, Clemons M. Amenorrhea, menopause, and endocrine therapy for breast cancer. BMJ. 2009. 339:b4261.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download