Abstract
Purpose
To investigate the clinical use of low-dose multidetector row computed tomography (MDCT) for staging of invasive breast cancers with patients in the prone position.
Methods
Three hundred twenty-two patients with 334 pathologically-verified breast cancers had low-dose MDCT breast imaging in the prone position for tumor staging before treatment between May 2006 and June 2010. We designed an additional computed tomography table pad with a hole for prone positioning. Patients lay prone on the table pad and the breasts were positioned within the rectangular hole. We obtained dynamic breast imaging from the lower neck to the lung base with the following parameters: 120 kVp, 50 mAs, and 3-mm reconstruction intervals. We evaluated the extent of the primary tumor, lymph nodal status, and distant metastasis in lung or bone, then assessed tumor staging based on the TNM classification of breast cancer. The assessed staging compared to the pathologic results for diagnostic accuracy.
Results
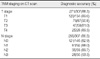
Among the 334 invasive breast cancers, the overall diagnostic accuracy of tumor staging was 88.3% and the accuracy values of each tumor stage were 89.6% in T1, 90.8% in T2, 81.0% in T3, and 89.3% in T4. The overall diagnostic accuracy of lymph nodal staging was 86.3% and the accuracy values in each nodal stage were 82.9% in N0, 88.0% in N1, 89.7% in N2, and 93.3% in N3. Based on breast computed tomography scans, we detected distant metastases in 30 cases (7 lungs, 10 bones, 7 lungs and bones, and 6 livers).
It has been reported that contrast-enhanced computed tomography (CT) is useful in the initial staging of breast cancer and detecting tumor recurrence after treatment.(1-7) Breast CT includes imaging of the breast as well as the lymph nodes located in the axillae, internal mammary and supraclavicular chains, lungs, and bones. Thus, the entire thorax can be evaluated in patients with breast cancer. However, the use of breast CT has been limited because of potential radiation hazards and image quality. Most studies involving breast CT have been conducted using standard doses with patients placed in the supine position, as based on the procedures used for conventional chest CT scanning.
Low-dose CT can reduce radiation exposure to the breast. A preliminary study using low-dose breast CT by Seo et al.(7) demonstrated satisfactory results for tumor staging in breast cancer. To improve breast image quality, multi-detector row CT (MDCT) and placement of the patients in the prone position might be helpful. MDCT obtains multiple CT data sets with each rotation of the X-ray tube; thus, the scan time is faster compared to the use of a conventional spiral CT scanner and MDCT provides greater temporal and spatial resolution of the images. The prone position is routinely used for breast magnetic resonance imaging (MRI). Breast MRI is obtained in a patient placed in the prone position by the use of a breast coil with both an integrated compression mechanism and appropriate spread of breast tissues. However, breast CT has been performed on patients placed in the supine position in most previous studies, as a specially designed CT table is required for placement of patients in the prone position, with special requirements, such as the use of a breast coil for breast MRI. We have designed an additional table pad that is placed on the standard CT table for patients undergoing CT imaging of the breast in the prone position.
The purpose of this study was to investigate the clinical use of low-dose MDCT for tumor staging of invasive breast cancers in the prone position.
The Institutional Review Board of our hospital approved this study. We used a 64-channel MDCT scanner (Brilliance 64-channel; Philips Medical Systems, Cleveland, USA). The scanning parameters were as follows: 120 kVp, 50 mAs, 16×0.625-mm collimation, 3-mm slide thickness, and 34-cm field of view. Before scanning the patients, we measured the CT dose index (CTDI100) for assessment of radiation dose using a standard adult CT body phantom (Model 76-415; Nuclear Associates, New York, USA). The CTDI100 values were measured at all 4 peripheral holes of the phantom and the values ranged from 2.01-4.08 mGy.
For breast CT scanning in the prone position, we made an additional table pad and placed the pad on the standard CT table (Figure 1A). The designed table pad was made of high-density polyfoam that was supportive and comfortable. The additional table pad had two parts (head and body). The head part was for positioning the head and the body part was for the chest and abdomen. The total size of the pad was 62 cm in width and 109 cm in length with maximum depth of 12 cm depth. For a comfortable patient position, the edge of the body part was gradually decreased to 5 cm in depth and was smoothed, and cotton fabric covered the pad. At the body part, the pad had a rectangular hole to position the breast that was 42 cm in width and 20 cm in length. The edges of the rectangular hole were also smoothed for patient comfort.
Patients lay prone on the table pad and the patient's breasts were positioned within the rectangular hole (Figure 1B). The patient raised both arms and relaxed, then turned their head to the right or left. We checked the patient's position and level of comfort, then scanned the patient from the level of the lower neck to the lower edge of the liver. For dynamic scanning, intravenous non-ionic contrast material was administrated and three phase images were obtained before, and 90 and 300 sec after a rapid injection of contrast material.(7) Three hundred twenty-two patients who had pathologically-verified breast cancers had low-dose MDCT breast imaging in the prone position for tumor staging before treatment between May 2006 and June 2010. We excluded patients who had surgical excision within 6 months before breast CT scanning, and patients who were lactating or pregnant. All of the patients were women and their ages ranged from 34-82 yr (mean, 47.8 yr). Twelve patients had bilateral invasive breast cancers and we assessed tumor staging for each breast according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual.(8)
Two radiologists reviewed the mammographic and ultrasound images of all patients, then evaluated CT images for tumor staging by consensus. Two radiologists assessed image quality of the low-dose MDCT images in the prone position in terms of tumor staging, then graded the images as follows: satisfactory, moderate, or poor.
We determined the stage for the primary tumor (T stage) and the regional nodal status (N stage) based on the newly revised AJCC Cancer Staging Manual.(8) We defined a positive breast lesion on CT images as a breast lesion that was focally-enhanced after contrast injection. We evaluated the changes in the skin and chest wall for assessment of the T stage. We measured the maximum size of the enhancing lesion with the images obtained 90 sec after an injection of contrast material. If the lymph node in the axilla had no internal fatty tissue and enhancement on post-contrast CT images, we considered a positive lymph node to be N1 stage. If the enhancing axillary node had indistinct margins and infiltration into the axillary fat or ipsilateral internal mammary node enlarged, we considered a positive lymph node to be N2 stage. If the positive enhancing lymph node was located in infra- or/and supra-clavicular areas, we considered the positive lymph node to be N3 stage. We also evaluated the presence or absence of lung masses or pleural effusions, osteolytic or obsteoblastic bone lesions in all of the thoracic bones, or hepatic masses. In 116 patients who underwent neoadjuvant chemotherapy before surgery, we obtained CT images after completion of neoadjuvant therapy and assessed these images according to the yp TNM classification.(8) For example, ypT1 stage was considered to be T1 stage in this study. We compared the staging on breast CT scan with the pathologic findings after surgery. If a patient had simultaneous bilateral primary cancers, each cancer was staged as a separate primary cancer in a separate organ and if a patient had multiple simultaneous ipsilateral primary cancers, the T stage was assessed in the largest tumor, according to the TNM rules.(8) The tumor stages assessed by CT scan were compared with the pathologic findings after surgery to determine the diagnostic accuracy of the low-dose breast MDCT images.
Two hundred ninety-six of 332 patients (91.9%) were assessed satisfactorily by the 2 radiologists (Figures 2, 3, 4, 5). Twenty-three patients (7.1%) had moderate-quality assessments because ring artifacts were detected, especially in the upper thorax; however, the ring artifacts did not interfere with the evaluation of the CT images by the radiologists. The remaining 3 patients (0.9%) had poor image quality owing to ring artifacts and image noise.
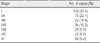
Table 1 demonstrates the TNM stages of all 334 invasive breast cancers in the 322 patients. Most patients (68.2%) had early invasive breast cancers, stage I in 102 patients (30.5%) (Figure 2) and stage II in 126 patients (37.7%). Advanced invasive breast cancers (stage III or IV) (Figures 3, 5) were diagnosed in 106 patients (31.7%).
Table 2 shows the comparison between TNM staging on breast CT scan and pathologic staging in patients with invasive breast cancers. Twenty-seven of 30 patients with distant metastases did not undergo surgical excision, thus pathologic T and N stages were not assigned (Figures 3, 5). We classified these 27 patients as Tx and Nx, according to the AJCC Cancer Staging Manual.(8) The overall diagnostic accuracy of the T stage was 88.3%, and the accuracy values of each tumor stage were 89.6% in T1, 90.8% in T2, 81.0% in T3, and 89.3% in T4. The overall diagnostic accuracy of lymph nodal staging was 86.3%, and the accuracy values in each nodal stage were 82.9% in N0, 88.0% in N1, 89.7% in N2, and 93.3% in N3. Twenty-three of 146 cancers (15.7%) with N0 stage were over-staged as N1 stage and 2 (1.4%) were assessed as N2 stage.
Distant metastatic lesions were found on breast CT scans in thirty patients, lungs (n=7), bones (n=10), lungs and bones (n=7), and liver (n=6) (Figure 5). In 14 patients with lung metastases, all metastatic lung lesions were detected on breast CT scans, and only 2 lung lesions were detected on chest radiographs (14.3%). Most bone lesions were located in the thoracic spines (n=6), ribs (n=3), sternum (n=4), or multiple bones (n=4).
Tumor staging of invasive breast cancers are important to determine whether or not the cancers are operable or inoperable, to design a treatment plan, and to predict prognosis. For tumor staging of invasive breast cancers, we should evaluate breasts, lymph nodes, and other organs that are frequent metastatic sites, such as bones, liver, or lungs.(8,9) For assessment of the T or N stage, we use physical examinations and radiologic findings on mammography, ultrasonography, or breast MRI. We should evaluate lymph nodes in the internal mammary, supra- or infra-clavicular areas, and axillae. Mammography or ultrasonography is limited in evaluating these lymph nodes, thus we need other imaging modalities to include these nodes. Clinically, breast MRI and conventional chest CT scans are used for this purpose. Breast MRI can evaluate a wider field of view, and supraclavicular and internal mammary lymph nodes; however, breast MRI cannot include lung or bone, and frequent metastatic sites. In addition, breast MRI has variable specificities (20-40%) and requires a long scan time.(10-12) In addition, inhomogeneous fat saturation of MRI also interferes with the evaluation of lymph nodal status, and patients with cardiac pacemakers or metallic implants cannot undergo MRI.
A CT is a good method by which to evaluate the entire thorax and liver for tumor staging in patients with invasive breast cancer. Breast CT imaging offers the ability to obtain three-dimensional anatomic detail and the procedure is more comfortable for patients as there is no breast compression and a short scan time, as compared to the use of mammography or breast MRI. However, even though breast CT imaging has these advantages, the use of breast CT imaging has been limited due to image quality and radiation exposure concerns. In terms of image quality, we considered the patient's position to fully spread the glandular breast tissues. Previous breast CT studies have placed the patients in the supine position. However, the use of the prone position eliminates superimposition of breast tissues and summation artifacts, and can improve the detection of lesions. Placement of patients in the prone position for breast CT imaging has been used for dedicated breast CT scanning.(13,14) The dedicated breast CT unit uses a cone-beam detector system and a specially designed CT unit, and includes only breast tissues in the field of view, as with mammography. Dedicated breast CT is a promising imaging modality for the breast; indeed, Lindfors et al.(13) has reported that the initial clinical experience using a dedicated breast CT was equal to the use of screen-film mammography for visualization of breast lesions. However, the use of dedicated breast CT has been limited as the modality requires a specially designed CT unit. Moreover, dedicated breast CT examines only breast tissue and other thoracic organs, such as the axillae, chest walls, lungs or bones, cannot be evaluated. In patients with invasive breast cancer, evaluation of other thoracic organs is required for assessment of tumor staging before treatment, detection of tumor recurrence, and follow-up treatment response.
To obtain breast CT images in the prone position, a specially designed CT table pad is necessary to prevent the breast tissues from compressing and folding on the standard CT table. We made an additional table pad with a rectangular hole for the prone position, then placed the pad on a standard CT table. The patient's breasts are placed in the hole and this method is similar to breast coils for a MRI examination. This hole allows for breast CT scanning with fully unfolded and extended breast tissues. The additional table pad was made with polyfoam. Polyfoam is easily obtained commercially and is both supportive and comfortable. To reduce radiation exposure, we used low-dose CT parameters (low tube voltage and current). Most of the CT studies on the breast have been performed using standard doses as for conventional chest CT imaging, in which the administered doses have been high. A study on breast dynamic MDCT using a standard-dose has reported that the measured radiation dose at the skin surface of the breast for a single breath-hold acquisition is 26 mGy.(15) We used a 120 kVp tube voltage and 50 mAs tube current to reduce the radiation dose. We measured the radiation dose using a CT body dose phantom to estimate the superficial radiation dose before the patient's examination. The measured doses were 2.01-0.48 mGy. Therefore, even though three-phase dynamic scans with low-dose parameters were performed, the total radiation dose was lower as compared to a singlephase standard-dose CT scan. Furthermore, in the current study, most of the patients (91.9%) had satisfactory image quality for diagnosis, even though the radiation dose was reduced.
In terms of tumor staging, our results demonstrated that low-dose MDCT in the prone position had high values of diagnostic accuracy for T and N stagings (88.3% and 86.3%). For T staging, 36 (11.7%) of 307 cases had inaccurate staging with breast CT scan when compared with pathologic findings. Among these 36 cases, there were two false negative cases. Two cases of the T1 breast cancers were not detected on CT scan because of insufficient enhancement. Twenty-five (83%) of 36 cases with inaccurate T staging on breast CT scans were over-staged and the remaining nine (25%) cases were down-staged. Over-staging was caused by overestimated tumor size, skin thickening, muscular invasion, or nipple invasion. In patients with thin breasts or bleeding after core biopsy, margins of tumors were indistinct and attached to the skin or muscular layers, thus the T stages were over-assessed. In N staging, 23 cases with no nodal involvement were considered to be positive lymph nodes on CT scans. Cortical thickening without fatty hila and enhancing nodes were assessed as positive nodes in this study. Some benign reactive lymph nodes can be enhanced and have scant fatty hila. Two patients with rheumatoid arthritis had multiple, large benign reactive nodes in the axillae. To improve diagnostic accuracy of breast CT scan, we should obtain high contrast and high quality images. For this purpose, the method of intravenous injection of contrast media for dynamic scan can be modified. High dose or rapid injection time of contrast media may be improve contrast between hypervascular breast lesions and normal glandular tissues on breast CT scan, thus, increase diagnostic accuracy of breast cancers. We hope further study about breast CT technique would be performed to improve image quality for breast CT imaging.
In local staging of breast cancers, detection of multiple synchronous breast cancers and evaluation of lymph nodes are very important. Mammography, ultrasound, and breast MRI have been used for local tumor staging. Breast MRI offers the highest sensitivity for detection of multiple breast cancers.(16,17) Previous studies about Breast MDCT demonstrated that the sensitivity and specificity of extensive intraductal components and multiple breast cancers were 76-91% and 89-91%.(1,18) Additionally, Nakahara et al.(19) concluded that 3D helical CT may be an alternative to 3D MRI for preoperative assessment of breast cancer in their comparative study between breast CT and MRI. Breast CT may improve overestimation of breast MRI for tumor staging based on their study.(19) A recent study by Taira et al.(20) demonstrated that preoperative breast CT was useful for detection of clinically and mammographically occult multiple breast cancers and the discovery rate of multiple breast lesions was 6%. Therefore, a comparative study in the same population is required in near future to conclude whether MRI or CT is superior for detecting mammographically occult multiple breast lesions. For evaluation of lymph nodal status, a recent study in a small population demonstrated presence of axillary lymph node with no fatty hilum on MRI correlated with pathologic node positivity.(21) However, there are currently no widely accepted MR criteria for the determination of axillary nodal status in breast cancer patients. Ultrasonographic examination has been shown to be moderately sensitive (48.8-87.1%) and fairly specific (55.6-97.3%) for the diagnosis of nonpalpable axillary metastases in a systemic review.(22) The diagnostic accuracy of N staging using low-dose breast MDCT in the current study was 86.3% and this result is satisfactory when compared with other imaging modalities such as ultrasonography or MRI.
There were some limitations in the current study. First, two radiologists subjectively graded the image quality of the breast CT scans. Quantitative and objective evaluation of image quality is necessary in a large population in a future study. Second, we measured radiation doses before the patient's examination with a body CT phantom and we did not measure radiation dose for each patient. There is no recommended guideline about radiation doses for breast CT scanning.
The American College of Radiology recommends an average glandular dose of ≤3 mGy for standard screening mammography, and thus, the radiation dose would be effectively 6 mGy or less for routine two-view mammography. When measured radiation doses administered to the CT phantom at 120 kVp and 50 mA in the current study were compared with recommended dose for mammography, a CTDI100 of ≤6 mGy was obtained (2.01-4.08 mGy).(23) Even though the measured CTDI100 values with the CT phantom in this study were low, the real radiation dose for each patient during breast CT scanning should be different. However, Hurwitz et al.(24) measured the breast radiation doses during chest CT scanning using anthropomorphic phantoms those were similar to human thorax and provided a dose savings of 55% to the breast at 120 kVp. Therefore, though we did not measure the exact radiation doses for each breast, radiation exposure to the breasts at 120 kVp and 50 mAs that we used in the current study must decrease the radiation doses for the breasts during the standard chest CT scanning. Further study would be needed for measurement of the radiation exposure to the breast glandular tissues during breast CT scanning because CTDI values can be variable according to CT imaging parameters and patient characteristics. CT image parameters include scanner geometry, tube voltage and currents, scanning mode, collimation, table speed, pitch, and gantry rotation time. However, we only considered tube voltage and current, and did not evaluate radiation dose according to the remaining parameters. The potential radiation risks in patients undergoing CT are due to stochastic effects.(25) The probability of stochastic effects depends on the amount of absorbed radiation. The breast is one of the radiosensitive organs and absorbed radiation dose is related with carcinogenesis.
Figures and Tables
 | Figure 1The additional computed tomography (CT) table pad and patient positioning. (A) A specially designed CT table pad is placed on the standard CT table for breast scanning in the prone position. The additional table pad is made with high-density polyfoam and has a rectangular hole for positioning of the breast. (B) The patient lies prone on the table pad and the breasts are positioned within the rectangular hole. The patient raises both arms and turns their head to the side. |
 | Figure 2A 47-yr-old woman with a left invasive ductal carcinoma. Dynamic computed tomography (CT) images are obtained precontrast (A) and after a 90-sec (B) and 300-sec delay (C) following contrast injection. The CT images show the presence of an indistinct irregular mass (arrows). The mass shows peak enhancement on the 90-sec delayed image (B) and washout on the 300-sec delayed image (C). On the pathologic examination, the size of the tumor measured 11 mm and the lymph nodes were negative for metastases. |
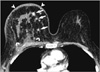 | Figure 3A 40-yr-old woman with a right inflammatory carcinoma. A 90-sec delayed computed tomography image shows that the right breast is enlarged and has multiple enhancing tumors (arrows). The overlying skin is thickened (arrowheads) and the pectoralis muscle is swollen (curved arrows) due to tumor infiltration. |
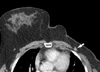 | Figure 4A 52-yr-old woman who underwent a mastectomy of the left breast for an invasive ductal carcinoma. An indistinct, irregular-shaped mass (arrow) is seen at the mastectomy site. The mass was pathologically-verified as a recurrent carcinoma. |
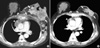 | Figure 5A 58-yr-old woman with an invasive ductal carcinoma who received chemotherapy. (A) The initial computed tomography (CT) image shows that an advanced tumor (arrows) of the left breast has invaded the skin and chest wall. Axillary and mediastinal lymph nodes (arrowheads) are enlarged and the sternum (curved arrows) is destroyed from metastases. (B) A follow-up CT image obtained 3 months after chemotherapy demonstrates that the primary tumor, metastatic nodes, and sternum were much improved. |
References
1. Akashi-Tanaka S, Fukutomi T, Miyakawa K, Uchiyama N, Tsuda H. Diagnostic value of contrast-enhanced computed tomography for diagnosing the intraductal component of breast cancer. Breast Cancer Res Treat. 1998. 49:79–86.

2. Uematsu T, Sano M, Homma K, Makino H, Shiina M, Kobayashi S, et al. Staging of palpable T1-2 invasive breast cancer with helical CT. Breast Cancer. 2001. 8:125–130.

3. Inoue T, Tamaki Y, Hamada S, Yamamoto S, Sato Y, Tamura S, et al. Usefulness of three-deminsional multidetector-row CT images for preoperative evaluation of tumor extension in primary breast cancer patients. Breast Cancer Res Treat. 2005. 89:119–125.

4. Hagay C, Cherel PJ, de Maulmont CE, Plantet MM, Gilles R, Floiras JL, et al. Contrast-enhanced CT: value for diagnosing local breast cancer recurrence after conservative treatment. Radiology. 1996. 200:631–638.

5. Cheng JC, Cheng SH, Lin KJ, Jian JJ, Chan KY, Huang AT. Diagnostic thoracic-computed tomography in radiotherapy for loco-regional recurrent breast carcinoma. Int J Radiat Oncol Biol Phys. 1998. 41:607–613.

6. Kang DK, Kim MJ, Jung YS, Yim H. Clinical application of multidetector row computed tomography in patients with breast cancer. J Comput Assist Tomogr. 2008. 32:583–598.

7. Seo BK, Pisano ED, Cho KR, Cho PK, Lee JY, Kim SJ. Low-dose multidetector dynamic CT in the breast preliminary study. Clin Imaging. 2005. 29:172–178.
8. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 2010. 7th ed. New York: Springer-Verlag.
9. NCCN guidelines for treatment of cancer by site. National Comprehensive Cancer Network. accessed Nov 10, 2010.
http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
10. Obdeijn IM, Kuijpers TJ, van Dijk P, Wiggers T, Oudkerk M. MR lesion detection in a breast cancer population. J Magn Reson Imaging. 1996. 6:849–854.

11. Boné B, Péntek Z, Perbeck L, Veress B. Diagnostic accuracy of mammography and contrast-enhanced MR imaging in 238 histologically verified breast lesions. Acta Radiol. 1997. 38:489–496.

12. Heywang-Köbrunner SH, Viehweg P, Heinig A, Kuchler C. Contrastenhanced MRI of the breast: accuracy, value, controversies, solutions. Eur J Radiol. 1997. 24:94–108.

13. Lindfors KK, Boone JM, Nelson TR, Yang K, Kwan AL, Miller DF. Dedicated breast CT: initial clinical experience. Radiology. 2008. 246:725–733.

14. Boone JM, Nelson TR, Lindfors KK, Seibert JA. Dedicated breast CT: radiation dose and image quality evaluation. Radiology. 2001. 221:657–667.

15. Inoue M, Sano T, Watai R, Ashikaga R, Ueda K, Watatani M, et al. Dynamic multidetector CT of breast tumors: diagnostic features and comparison with conventional techniques. AJR Am J Roentgenol. 2003. 181:679–686.

16. Kuhl C, Kuhn W, Braun M, Schild H. Pre-operative staging of breast cancer with breast MRI: one step forward, two steps back? Breast. 2007. 16:Suppl 2. S34–S44.

17. Lim HI, Choi JH, Yang JH, Han BK, Lee JE, Lee SK, et al. Does preoperative breast magnetic resonance imaging in addition to mammography and breast ultrasonography change the operative management of breast carcinoma? Breast Cancer Res Treat. 2010. 119:163–167.

18. Uematsu T, Sano M, Homma K, Shiina M, Kobayashi S. Three-dimensional helical CT of the breast: accuracy for measuring extent of breast cancer candidates for breast conserving surgery. Breast Cancer Res Treat. 2001. 65:249–257.

19. Nakahara H, Namba K, Wakamatsu H, Watanabe R, Furusawa H, Shirouzu M, et al. Extension of breast cancer: comparison of CT and MRI. Radiat Med. 2002. 20:17–23.
20. Taira N, Ohsumi S, Takabatake D, Hara F, Takashima S, Aogi K, et al. Contrast-enhanced CT evaluation of clinically and mammographically occult multiple breast tumors in women with unilateral early breast cancer. Jpn J Clin Oncol. 2008. 38:419–425.

21. Mortellaro VE, Marshall J, Singer L, Hochwald SN, Chang M, Copeland EM, et al. Magnetic resonance imaging for axillary staging in patients with breast cancer. J Magn Reson Imaging. 2009. 30:309–312.

22. Alvarez S, Añorbe E, Alcorta P, López F, Alonso I, Cortés J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006. 186:1342–1348.

23. American College of Radiology. ACR Practice Guideline for the Performance of Screening Mammography. 2006. Reston: American College of Radiology;217–225.
24. Hurwitz LM, Yoshizumi TT, Goodman PC, Nelson RC, Toncheva G, Nguyen GB, et al. Radiation dose savings for adult pulmonary embolus 64-MDCT using bismuth breast shields, lower peak kilovoltage, and automatic tube current modulation. AJR Am J Roentgenol. 2009. 192:244–253.

25. 1990 Recommendations of the International Commission on Radiological Protection. Ann ICRP. 1991. 21:1–201.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download