Abstract
Purpose
A significant improvement of imaging using synchrotron radiation (SR) is obtained by introducing phase-contrast technique. This technique provides greatly enhanced contrast and good soft tissue discrimination with high spatial resolution. The aim of this study was to observe microstructures of pathologic breast specimens including invasive breast cancer using phase-contrast technique with SR and to evaluate the feasibility of phase-contrast imaging in clinical application.
Methods
Phase-contrast microscopic image of normal breast tissue and the images of various breast diseases such as fibrocystic change, ductal carcinoma in situ, invasive ductal carcinoma, Paget's disease were obtained using hard X-ray microscopy with an 11.1 keV monochromatic beam from SR source and CsI (TI) scintillation crystal. Zernike phase-shifter was adapted for phase-contrast hard X-ray microscopy. The visual image was magnified 20 times by microscopic objective lens and captured using a full frame charge-coupled device camera. Obtained images were compared with corresponding histopathologic findings in the optical microscopy.
Results
The SR images of various breast diseases were obtained with a good contrast and high visibility by phase-contrast technique. It was possible to observe the microstructures with high spatial resolution down to the micron region. The characteristic features of each disease were consistent with the histopathologic findings of corresponding sample and the images of breast cancer and the other diseases were distinct from each other.
Conclusion
Using phase-contrast technique, SR images of various breast diseases including breast cancer were obtained. These images were comparable with standard histopathologic findings and showed different features for each disease. The results suggest that phase-contrast microscopic imaging with SR has potential as a diagnostic tool and also its clinical application is feasible, especially in breast imaging.
Breast cancer is the most common neoplasm in women and the leading cause of cancer-related deaths worldwide.(1) The prognosis of early breast cancer is relatively favorable, so early detection and treatment of breast cancer can reduce the breast-cancer mortality rate.(2) The mammography screening allows much earlier detection and diagnosis of breast cancer, and many imaging methods such as ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography-CT were used for diagnosis of breast diseases. Recently, new techniques such as digital mammography, high-resolution ultrasonography, computer aided diagnosis have been developed, and many studies using synchrotron radiation (SR) are in progress to optimize image quality and improve the diagnostic capabilities.
SR is electromagnetic radiation generated by a synchrotron. When high-energy particles are in rapid motion, including electrons forced to travel in a curved path by a magnetic field, SR is produced.(3) The properties of synchrotron X-rays are quite different from those of conventional X-ray sources based on the Röntgen mechanism. Synchrotron sources provide highly collimated and powerful beams with linear polarization and make it possible to show the internal structures of biological soft tissues with high spatial resolution under low radiation dose and may offer greatly enhanced image quality over conventional imaging techniques.(4)
In recent years, several imaging techniques based on SR such as phase-contrast imaging, diffraction-enhanced imaging (DEI) have been developed and extensively studied for potential medical applications.(5,6) In particular, SR mammography,(7) phase-contrast mammography,(8,9) high-contrast mammography using a DEI technique(10) and DEI-CT(11) were introduced into breast imaging, and in our previous study, we were able to observe the detailed internal structures of normal breast tissue, fibroadenoma and breast cancer tissue by phase-contrast hard X-ray microscopy with SR.(12,13)
In this study, we obtained phase-contrast microscopic images of normal breast tissue and various pathological breast specimens such as fibrocystic change (FCC), ductal carcinoma in situ (DCIS), invasive ductal carcinoma (IDC), Paget's disease using SR, and compared these images with corresponding histopathologic findings. Also we discussed the feasibility of phase-contrast microscopic imaging with SR as a diagnostic imaging technique.
The specimens were chosen at random from the resected tissues of the patients who underwent surgery for various breast diseases such as FCC, DCIS, IDC, and Paget's disease at Daegu Catholic University Medical Center in Daegu, Korea. We selected 2 tissues for 1 disease entity each.
All tissues were routinely fixed in 10% neutral buffered formalin and embedded in paraffin blocks. The normal breast tissue was taken from the normal portion of the resected tissue with DCIS. The prepared tissue blocks were then sectioned at a profer thickness ranging from 5 µm to 10 µm. Some of these formalin-fixed sections were attached on Kapton film for each disease to get the X-ray images.
The other sections, adjacent to the samples which were prepared for X-ray imaging were layered on glass slides and stained with hematoxylin and eosin (H&E) and then, histologically examined under an optical microscope by a pathologist.
Experiments were performed at the bending magnet beamline 1B2/microprobe of the Pohang Light Source (PLS) in Pohang Accelerator Laboratory (PAL), in Pohang, Korea, which is a third-generation SR facility with an operating energy of 2.5 GeV.
In this experiments, Bragg diffraction from crystals was used to monochromatise the white beam provided by the bending magnet source. The schematic set-up of this experiments is shown on Figure 1. The photon energy was set at 11.1 keV, and the sample was positioned 25 m away from the synchrotron X-ray source. After injection of electron, the X-rays pass through a double multilayer monochromator consisting of a pair of W/B4C multilayers with a 2 d of 50 Å. Zernike phase-shifter was adapted for phase-contrast X-ray microscopy. The X-ray images of the specimens were converted into a visual one on the surface of a 10 µm-thick CsI (TI) scintillation crystal which was placed at a distance of 5 cm from the specimen. This visible images were further enlarged by a 20x microscopic objective lens and captured by a full-frame charge-coupled device camera (CCD camera; Megaplus II ES 2001; Redlake, Pasadena, USA) after an additional 10 fold digital magnification. The output of the monochrome CCD camera was fed into a video cassette recorder, and later the recorded video tapes were converted into mpg files as necessary.
A field of view is determined by the size of the CCD chip and the optical magnification. In our images, the CCD camera had a spatial resolution of 648×484 pixels and the field of view was 640×480 µm2. As the field of view was limited in this preliminary experiment, the whole image of each specimen was reconstructed with mapping the pieces of images of a specimen.
The X-ray microscopic image of normal breast tissue and the images of various breast diseases such as FCC, DCIS, IDC, Paget's disease were obtained with a good contrast and high visibility by phase-contrast technique. Total magnifying power of this microscope was 200 times in the end. Internal structures visible in SR images were compared with corresponding histopathologic findings. The SR image of normal breast tissue showed well defined terminal duct-lobular unit (TDLU) surrounded by trabeculae of collagen bundles and mature fat cells (Figure 2). And fine structures of small acini and interacinar spaces of the TDLU were visible in SR image also. Insignificant pathophysiologic changes of breast such as macroscopically visible cyst, fatty changes were shown in SR images of FCC and these features were consistent with the histopathologic findings of FCC (Figure 3).
The SR images of DCIS revealed various structures including prominent and intact periductal basement membrane, which is a hall mark of DCIS lesion, and stippled fine microcalcification, and a shadow of intense peritumoral inflammatory cell infiltration. These features correspond to the histopathologic findings of the DCIS, but the minute change of each cancer cell showing the features of cancer tissue such as cellular differentiation and nuclear grade were not identified well (Figures 4, 5).
The SR images of IDC showed the features of the breast cancer including severe fibrosis of stroma with irregular infiltration of tumor cells and infiltration of tumor cells into adjacent fat tissues, and these features are comparable with that of conventional histopathological examination by light microscopy (Figure 6). But, lymphocytes nests infiltrating stroma and fine microscopic features of breast cancer tissues were not imaged well with this technique.
SR images of Paget's disease exhibit lacuna shaped intraepidermal Paget cells of the nipple clearly. There was a close correspondence between the SR images and the histopathologic findings Paget's disease (Figure 7). The infiltration of inflammatory cells and profuse collagen strands were well recognized even in the low power SR images, but the squamous epithelium of epidermis was not.
The results of this study showed that the microstructures of various breast diseases such as FCC, DCIS, IDC, and Paget's disease could be identified by the phase-contrast microscopic imaging with synchrotron radiation and these images had correlation with the histopathologic findings.
Microscopic findings such as infiltrating tumor cells into fat tissue, irregular infiltration of threads like collagenous stroma and fibrotic change of stroma are characteristic features of breast cancer(14) and these were identified in the SR images and in the corresponding histopathologic findings of breast cancer tissues as well. Histologically, DCIS is a unicentric proliferation of epithelial cells with cytological features of malignancy within parenchymal structures of the breast and is distinguished from invasive carcinoma by the absence of stromal invasion across the basement membrane.(15) In Paget's disease, the Paget cell is a large pale-staining cell with round or oval nuclei and large nucleoli, and the cells are between the normal keratinocytes of the nipple epidermis, occuring singly in the superficial layers and in clusters toward the basement membrane.(16) These characteristic features of each pathologic tissue were well recognized in this study. But, the minute changes such as lymphocyte infiltration, cellular differentiation, nuclear grade were not visualized well in the phase-contrast images, although the images were acquired with high resolution of 1.2 µm. This may due to the overlapping structures with varying amount of phase-contrast and insufficient edge-enhanced contrast in the microstructures of the cell.
It is remarkable that the findings of SR images of breast cancer tissues were different from those of FCC. The former revealed the findings consistent with malignancy but the latter was suggestive of benign lesion. From these results, it has a clinical significance that phase-contrast microscopic imaging with SR can be used to diagnose various diseases of the breast at early stage and to differentiate breast cancer from the other benign disease also.
In addition, because X-ray penetrates objects, this technique makes it possible to visualize internal structures of a tissue without destruction and there is no need for tissue preparation as in optical microscopy.(17) So it is available for noninavasive microscopic imaging of intact tissue and has a potential to apply in vivo even for live patients. Although we examined only about 5 µm to 10 µm thick tissue sections, it is feasible to get the images of a whole tissue in the near future. The main reason for sectioning the tissues in this study was to use a sample thick enough, that an X-ray flux penetrates. An 11.1 keV monochromatic X-ray beam, the highest X-ray energy with maximum flux, was available from a monochromator at the current 1B2 beamline of PAL, but this energy is not able to penetrate a sample thicker than 2 mm. However, currently PAL is undergoing an upgrade project to increase injector energy up to 3 GeV, and to prepare a multipole-wiggler-type insertion device that will provide an enhance X-ray flux over a large energy range (20-60 keV) for biomedical imaging application. When this project is completed, it would be possible to obtain the images of a whole tissue as well as to study noninvasive in vivo imaging.
X-ray phase-contrast was reported in the middle of 1990s, and since then, phase-contrast X-ray imaging techniques have been extensively studied in recent years, especially for their biomedical applications.(5,6) Conventional X-ray imaging techniques utilize differences in sample absorption to yield image contrast and it has a limitation in differentiating soft tissues due to the weak attenuation contrast, however phase contrast techniques can provide great enhancements in the image, especially using synchrotron radiation sources.(18,19) Recently, the phase-contrast hard X-ray microscope using Zernike's method was found to give much a better contrast than the absorption contrast(20-22) and Youn et al.(23) developed a hard X-ray microscope with a submicrometer spatial resolution at the PLS, which was used in this study.
Phase-contrast hard X-ray microscopy is an imaging technique in which small phase shifts in the light passing through a transparent specimen are converted into amplitude or contrast changes in the image and the light source is hard X-ray (10-100 keV) using synchrotron radiation which can improve image contrast. In this study, a crystal monochromator was used to select a small energy band from the incident synchrotron radiation and Zernike phase contrast was applied to improve contrast. The spatial resolution was improved by increasing the magnification of the microscope objective lens looking at the CsI(Tl) scintillation crystal and the resulting resolution, 1.2 µm, was much higher than the 30 µm resolution from synchrotron mammography, and was enough to reveal fine internal structures.
Most of pathologic processes of breast diseases arise in the breast tissues including mammary duct, breast parenchyma and result in morphological changes such as increased collagen fibrils, fibrosis, cystic change, cell proliferation or infiltration. Identifying these morphologic and cellular features with phase-contrast hard X-ray microscopy has many potential in clinical application. Noninvasive diagnosis of breast cancer could be given in live patients, margins of excised tissue or confirmative diagnosis could be assessed intraoperative consultations at the time of surgical excision, determinations of adequacy in surgical extent could be confirmed after surgery and local recurrence of tumor could be detected in a postoperative follow-up image.
Recently other imaging techniques such as confocal scanning microscopy (CSLM),(24) optical coherence tomography (OCT),(25,26) have been studied to visualize internal structures of biological specimen. But the CSLM is not broadly applicable for intact, live animals due to animal opacity and size limitation and it allows for examinations near the surface only due to limitation of penetration depth less than 0.3 mm.(24) The OCT is currently used in the field of ophthalmology and dermatology, and is attracting interest among the medical community because it provides tissue morphology imagery at much higher resolution (better than 10 µm) than other imaging modalities such as MRI or ultrasound. However this technique is also limited to imaging 1 mm to 2 mm below the surface in biological tissue,(25) so it is ineffective at visualizing deep soft tissues. Phase-contrast hard X-ray microscopy, in the other hand, is useful for most of soft tissues since hard X-ray penetrates tissues regardless of thickness.
In general, phase-contrast hard X-ray microscopy offer projected 2D images on which tissue structures can be visualized. So structures at different depths would be superimposed on each other and the overlapping structures could be confused.(5) Therefore reconstruction of 3D images from 2D images of closely spaced slices of whole tissue, for instance, CT will need to be developed for accurate visual inspection. Actually we are studying the phase-contrast tomography technique using SR, and other studies of breast CT in early breast cancer detection have been investigated.(27,28)
Of course, for diagnostic radiography applications, the X-ray doses have to be carefully controlled and maximum limits have to be set to minimize the risk for the patient. Recent studies on breast tissue specimens have demonstrated that phase-contrast X-ray images were obtained at either similar or lower doses than used in conventional mammography.(5,7,29,30) Although the radiation doses delivered could not be measured in our study, it seems to be lower than conventional radiography since the monochromatic radiation was used, and accurate dosimetry and quantitative analysis will be needed further study. As mentioned before, currently PAL is undergoing an upgrade project to increase injector energy and when this project is completed, the radiation dose necessary for producing monochromatic SR X-ray images in the 20-60 keV energy range would be similar to that is delivered with the conventional grid apparatus.(7)
In fact, although phase-contrast images provide an improved contrast over attenuation-based images for soft tissues, it is difficult to interpret the images since practically no clinical experience has so far been available on how tumor characteristics and structures are represented in such phase-contrast images in human. So, for medical applications, additional studies are required to develop a comprehensive understanding of the correlation of phase-contrast images to histopathologic findings for the various conditions and disease of the breast. And the standard criteria for image interpretation are needed so that details visualized in the phase-contrast images can be assigned easily to known lesions.
The results and discussion in this study demonstrated that phase-contrast hard X-ray microscopy can identify the microstructures of breast cancer and other breast diseases, and the SR images can be directly correlated with the histopathology of the tissue sample. It is clear that further developments in SR imaging is needed to overcome the limitations in the current study, but the present results give some directions of medical application. We suggest that the phase-contrast hard X-ray microscopy has great potential as an imaging tool for clinical and research purpose in the near future.
Figures and Tables
 | Figure 1Schematic drawing of the experimental set-up. Monochromatic beam set at 11.1 keV (=50Å) irradiated the sample positioned 25 m away from the source. A visual signal on the surface of CsI (TI) scintillation crystal placed at a distance of 5 cm from specimen was magnified using a 20× microscopic objective lens and captured using CCD camera. |
 | Figure 2(A) Comparison of normal terminal duct-lobular unit (TDLU) structures of breast between synchrotron radiation (SR) image and (B) histologic section (H&E stain, ×40). SR image shows well defined TDLU surrounded by trabeculae of collagen bundles (*) and mature fat cells (arrow heads). Fine structures of small acini and interacinar spaces (arrow) of the TDLU are visible in SR image. |
 | Figure 3(A) Phase-contrast synchrotron radiation (SR) image and (B) optical microscopic image (H&E stain, ×40) of fibrocystic change. It shows cystic change (arrow) and fatty tissue (arrow head). 10 µm-thick formalin-fixed tissue was used and measured 1.3×0.9 mm in size. |
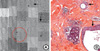 | Figure 4(A) Comparison of synchrotron radiation (SR) image and (B) histologic section (H&E stain, ×40) of low grade ductal carcinoma in situ lesion of cribriform pattern. The SR image well correlated to the histologic section with prominent periductal basement membrane (arrows) and stippled fine microcalcifications (black dots in left tumor nest, circle). Round fenestrae (*) of the tumor cell nests are highlighted in the SR image. |
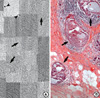 | Figure 5(A) Comparison of synchrotron radiation (SR) image and (B) histologic section of ductal carcinoma in situ lesion of solid and cribriform pattern (H&E stain, ×40). The SR image well correlates to the histologic section with prominent periductal basement membrane (arrows) and stippled fine microcalcifications (black dots in tumor nest, arrow head). Peritumoral inflammatory cell infiltration are highlighted in the SR image (circle). |
 | Figure 6(A) Phase-contrast synchrotron radiation (SR) image and (B) optical microscopic image (H&E stain, ×40) of breast cancer. It shows irregular tumor cell infiltration into adjacent fat tissue (arrows) and parenchyme with severe stromal fibrosis (arrow head). (C) Cords and individual infiltrating tumor cells in the desmoplastic stroma are seen in a high-power field view (H&E stain, ×400). The specimen was formalin-fixed breast cancer tissue, 10 µm thick and 0.9×0.8 mm in size. |
 | Figure 7(A) Phase-contrast synchrotron radiation (SR) image and (B) optical microscopic image (H&E stain, ×40) of Paget's disease. SR image shows lacuna shaped intraepidermal Paget cells (arrows) of nipple epidermis. In SR image, the Paget cells show large electron dense nuclei and electron lucent abundant cytoplasm with distinct cell outlines, and most squamous cells are indistinct in cell border. |
References
1. Housa D, Housová J, Vernerová Z, Haluzik M. Adipocytokines and cancer. Physiol Res. 2006. 55:233–244.

2. Jatoi I, Miller AB. Why is breast cancer mortality declining? Lancet Oncol. 2003. 4:251–254.
3. Margaritondo G, Meuli R. Synchrotron radiation in radiology: novel X-ray sources. Eur Radiol. 2003. 13:2633–2641.

4. Lewis RA, Rogers KD, Hall CJ, Hufton AP, Evans S, Menk RH, et al. Antonuk LE, Yaffe MJ, editors. Diffraction enhanced imaging: improved contrast, lower dose Xray imaging. Medical Imaging 2002: Physics of Medical Imaging, San Diego, USA. 2002. Bellingham: Society of Photo-optical Instrumentation Engineers;286–297.
5. Lewis RA. Meidcal phase contrast X-ray imaging: current status and future prospects. Phys Med Biol. 2004. 49:3573–3583.
6. Suortti P, Thomlinson W. Medical applications of synchrotron radiation. Phys Med Biol. 2003. 48:R1–R35.

7. Burattini E, Cossu E, Maggio C, Gambaccini M, Indovina PL, Marziani M, et al. Mammography with synchrotron radiation. Radiology. 1995. 195:239–244.

8. Arfelli F, Bonvicini V, Bravin A, Cantatore G, Castelli E, Palma LD, et al. Mammography with synchrotron radiation: phase-detection techniques. Radiology. 2000. 215:286–293.

9. Ingal VN, Beliaevskaya EA, Brianskaya AP, Merkurieva RD. Phase mammography: a new technique for breast investigation. Phys Med Biol. 1998. 43:2555–2567.
10. Liu C, Yan X, Zhang X, Yang W, Peng W, Shi D, et al. Evaluation of X-ray diffraction enhanced imaging in the diagnosis of breast cancer. Phys Med Biol. 2007. 52:419–427.

11. Fiedler S, Bravin A, Keyriläinen J, Fernández M, Suortti P, Thomlinson W, et al. Imaging lobular breast carcinoma: comparison of synchrotron radiation DEI-CT technique with clinical CT, mammography and histology. Phys Med Biol. 2004. 49:175–188.

12. Jeong YJ, Bong JG, Kim HT, Kim JK, Jheon SH, Youn HS, et al. Synchrotron radiation imaging of female breast tissues using phase contrast technique. J Breast Cancer. 2008. 11:40–44.

13. Park SH, Kim HT, Kim JK, Jheon SH, Youn HS. Hard X-ray microscopic imaging of human breast tissues. 2006. In : 9th International Conference on Synchrotron Radiation Instrumentation; Daegu: American Institute of Physics.
14. Stuart JS, Anthony JG. Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Pathology of invasive breast cancer. Diseases of the Breast. 2004. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;542–584.
15. Azzopardi JG. Azzopardi JG, Ahmed A, Millis RR, editors. Epitheliosis and in situ carcinoma. Problems in Breast Pathology. 1979. Philadelphia: Saunders;128.
16. Rosen PP. Rosen PP, editor. Paget's disease of the nipple. Rosen' Breast Pathology. 1997. Philadelphia: Lippincott-Raven;493–506.
17. Youn HS, Jung SW. Hard X-ray microscopy with Zernike phase contrast. J Microsc. 2006. 223(Pt 1):53–56.

18. Weon BM, Je JH, Hwu Y, Margaritondo G. Phase contrast X-ray imaging. Int J Nanotechnol. 2006. 3:280–297.

19. Socha JJ, Westneat MW, Harrison JF, Waters JS, Lee WK. Real-time phase-contrast X-ray imaging: a new technique for the study of animal form and function. BMC Biol. 2007. 5:6.

20. Kagoshima Y, Yokoyama Y, Niimi T, Koyama T, Tsusaka Y, Matsui J, et al. Hard X-ray phase-contrast microscope for observing transparent specimens. 2002. In : 7th International Conference on X-Ray Microscopy; Grenoble: European Synchrotron Radiation Facility.
21. Andrews JC, Almeida E, van der Meulen MC, Alwood JS, Lee C, Liu Y, et al. Nanoscale X-ray microscopic imaging of mammalian mineralized tissue. Microsc Microanal. 2010. 16:327–336.

22. Chen J, Yang Y, Zhang X, Antrews JC, Pianetta P, Guan Y, et al. 3D nanoscale imaging of the yeast, Schizosaccharomyces pombe, by full-field transmission X-ray microscopy at 5.4 keV. Anal Bioanal Chem. 2010. 397:2117–2121.

23. Youn HS, Baik SY, Chang CH. Hard X-ray microscopy with a 130 nm spatial resolution. Rev Sci Instrum. 2005. 76:023702.
24. Schiffhauer LM, Boger JN, Bonfiglio TA, Zavislan JM, Zuley M, Fox CA. Confocal microscopy of unfixed breast needle core biopsies: a comparison to fixed and stained sections. BMC Cancer. 2009. 9:265.

25. Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991. 254:1178–1181.

26. Fercher AF, Drexler W, Hitzenberger CK, Lasser T. Optical coherence tomography: principles and applications. Rep Prog Phys. 2003. 66:239–303.
27. Pani S, Longo R, Derossi D, Montanari F, Olivo A, Arfelli F, et al. Breast tomography with synchrotron radiation: preliminary results. Phys Med Biol. 2004. 49:1739–1754.

28. Lindfors KK, Boone JM, Nelson TR, Yang K, Kwan AL, Miller DF. Dedicated breast CT: initial clinical experience. Radiology. 2008. 246:725–733.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download