Abstract
Purpose
Phyllodes tumors (PTs) of the breast have been classified as benign, borderline, or malignant based on their histopathologic features. However, predicting clinical behavior based on these features has proven to be difficult given that local recurrence occurs in both benign and malignant PTs. Recurrence has been shown to mirror the histologic pattern of the primary tumor or to show dedifferentiation. The aim of this study was to assess the value of the histopathologic parameters, expression or mutation of c-Kit and platelet derived growth factor receptor alpha (PDGFRA) in predicting tumor recurrence.
Methods
Representative areas from 39 benign, 16 borderline, and 12 malignant PTs were selected for construction of tissue microarrays. Immunohistochemical analyses for p53, Ki-67, c-Kit, and PDGFRA were performed and SSCP-PCR analysis was carried out to identify mutations in exons 9, 11, 13, and 17 of the c-Kit gene and exons 12 and 18 of the PDGFRA gene. Clinicopathologic features, including tumor recurrence and margin status, were also evaluated.
Results
Of the 67 PTs, 11 cases (16.4%) recurred from 3 to 92 months following initial diagnosis (4 benign, 2 borderline, and 5 malignant). One benign PT case recurred as a borderline tumor and two borderline PT cases recurred as malignancies. Three patients died of malignant PT. No mutations of the c-Kit or PDGFRA genes were found and there was no statistically significant association of either p53 or p16 immunostaining with recurrent disease (p>0.05). However, histologic grade (p=0.033), margin status (p<0.001), Ki-67 (p=0.012), c-Kit (p=0.002), and PDGFRA (p=0.007) stromal immunopositivity were significantly correlated with recurrence.
Phyllodes tumors (PTs) are biphasic fibroepithelial neoplasms, characterized by a leaf-like architecture. They are composed of benign epithelial elements and a cellular, spindle cell stroma. These lesions account for less than 1% of all breast tumors.(1,2)
The behavior of PTs ranges from benign and locally recurrent to malignant and metastatic.(3) The clinical behavior has been difficult to predict based on histologic features. Histologically benign PTs have been known to metastasize, and histologically malignant PTs sometimes neither recur nor metastasize.(4,5) The current (2003) World Health Organization (WHO) classification of tumors of the breast separates PTs into benign, borderline, and malignant categories, mainly based on mitotic activity, margin type, stromal overgrowth, and cellular pleomorphism.(6) Recently ancillary diagnostic methods to identify PTs with potentially aggressive behavior have been reported. These have included immunohistochemical assessment of p53, Ki-67, and c-Kit.(7-11)
Both c-Kit and platelet derived growth factor receptor alpha (PDGFRA) belong to the platelet derived growth factor receptor (PDGFR) subfamily of tyrosine kinase receptors. Receptor tyrosine kinases have been extensively studied owing to their frequently abnormal activation in the development and progression of human cancers.(12) The c-Kit oncogene encodes the tyrosine kinase transmembrane receptor (CD117) protein. Gastrointestinal stromal tumors (GISTs), which have been characterized by the overexpression of c-Kit, show a spectrum of behaviors from benign to malignant, similar to PTs.(13-15) Recently, expression of both c-Kit and PDGFRA was observed in malignant PTs, although the impact of this recurrence was not addressed.(7-11,16)
The aim of this study was to assess the value of the histopathologic parameters, c-Kit and PDGFRA expression and mutation in predicting tumor recurrence.
Sixty seven PTs of the breast were diagnosed between January 1998 and December 2006 at the Department of Pathology, Chungnam National University Hospital and Eulji Hospital, Daejeon. This study was approved by the Institutional Review Board of Chungnam National University School of Medicine. According to histologic findings (Table 1), including the degree of stromal hypercellularity, cellular pleomorphism, mitotic activity, and tumor border, PTs were classified into benign, borderline, and malignant categories.(1,6) At least two hematoxylin and eosin (H&E) stained tumor sections were reviewed independently by three pathologists for verification of diagnosis. The resection margin was recorded as positive when tumor cells were located at the inked margin; as close when tumor cells were within 0.2 mm of the margin; and as negative when tumor cells were more than 0.2 cm from the margin (Figure 1). In vacuum-assisted excision cases, we examined every core of excised tissue and checked the margin status. Patient follow-up information was obtained from chart reviews.
Representative areas from 67 PTs were selected for construction of tissue microarrays using a 3 mm punch with two punches per case. Samples were arrayed in the recipient blank blocks. A total of 67 PTs were used. Immunohistochemical analyses for p53, Ki-67, c-kit, and PDGFRA were performed. Further, 4 µm sections were cut from the tissue microarray blocks and fished onto coated slides. The slides were then baked in an oven overnight at 55℃ to enhance adhesion. Deparaffinization in xylene and graded alcohol followed. Pre-diluted antibodies against Ki-67 (monoclonal; Zymed Lab., San Francisco, USA), p53 (monoclonal; DAKO, Copenhagen, Denmark), c-Kit (polyclonal; DAKO), p16 (polyclonal; DAKO), and PDGFRA (monoclonal; Santa Cruz Biotechnology Inc., Santa Cruz, USA) were obtained from commercial sources. Immunohistochemical staining protocols for each antibody are summarized in Table 2. Immunolocalization was performed using an LSAB kit (DAKO, Carpinteria, USA). All assays were carried out prospectively in an automated immunostainer (DAKO). Antibody-antigen reactivity was visualized using diaminobenzidine and counterstained with Meyer's hematoxylin.
The percentage of tumor cells was semiquantified manually by counting 500 stromal cells per case. Stains for p53 and Ki-67 were assessed in the cell nuclei, while c-Kit, p16, and PDGFRA were evaluated in the cytoplasm and cytoplasmic membrane. p53, c-Kit, p16, and PDGFRA were considered to be positive when more than 10% of stromal cells were positive. A high Ki-67 index was defined as nuclear staining in more than 10% of tumor cells. An ovarian serous carcinoma known to be positive for p53 and a c-Kit positive case of GIST were used as positive controls. For negative controls, the primary antibody was omitted with each batch run. Overall positive marker expression between tumors that did and did not recur was assessed.
Twenty-eight tumor samples (16 borderline tumors and 12 malignant tumors) were taken from formalin fixed, paraffin embedded (FFPE) tissue samples. Subsequently, H&E-stained 4 µm sections were reviewed under a microscope and areas rich in tumor cells were marked. Corresponding areas on unstained sections were then scraped from the slides using a scalpel blade. Tumor samples that contained as few non-neoplastic cells as possible (70-90% tumor cellularity) were collected. Tissue blocks were required to have at least 85% neoplastic cells. In order to meet this requirement, tumor areas were dissected from surrounding normal tissues. DNA samples from breast PTs and normal breast tissue pairs were prepared. A total of 3 microdissected 5 µm sections of FFPE tumor tissue were incubated at 52℃ for one or two days in 400 µL of DNA extraction buffer (0.25 µg/µL proteinase K)(Roche, Mannheim, Germany), 20 mM Tris/HCl, pH 8.3, 5 mM MgCl2, 100 mM KCl, 1% Tween-20, and 1% NP-40). The mixture was boiled for 10 min to inactivate the proteinase K, followed by phenol extraction for purification, and concentration using ethanol precipitation. The isolated DNA solution was quantified spectrophotometrically.
SSCP-PCR analysis for mutations in exons of 9, 11, 13, and 17 of the c-Kit gene, and exons 12 and 18 of PDGFRA was carried out, as previously described.(17,18) PCR amplification was performed in a total volume of 20 µL containing 500 ng of template DNA, one unit of ExTaq polymerase (Takara, Shiga, Japan), 1.25 mM dNTP, 15 pmole primer, and 2 µL of 1×reaction buffer. PCR cycles consisted of 5 min at 94℃ followed by 35 cycles for 30 sec at 94℃, 30 sec at 55℃, and 30 sec at 72℃, followed by one cycle for 7 min at 72℃.
Two microliters of PCR product was mixed with 6 µL of sample loading buffer containing 95% formide (deionized), 10 mM NaOH, 0.25% Bromophenol blue, and 0.25% Xylene cyanol. The samples were then denatured for 3 min at 100℃ and quickly chilled on ice. They were then loaded onto 12% polyacrylamide gel containing 1×sample buffer (33 mM Tris-sulfate, 7% Glycerol, pH 8.3), and were electrophoresed at 250 V. After electrophoresis the gels were disassembled from the glass plate, then stained using a Silver Stain Plus kit (BIO-RAD, Philadelphia, USA), followed by air drying.
Statistical analysis was carried out using SPSS software (PASW Statistics 17.0) (SPSS Inc., Chicago, USA). Histologic parameters predictive of recurrence were correlated with p53, Ki-67, c-Kit, p16, and PDGRA immunostaining results using a two-sided chi-square test or Fisher's exact test in cross tables. For multivariate analysis, immunohistochemical staining results were designated as binary variables (positive vs. negative). Multivariate analysis was also performed using binary logistic regression analysis. A two-tailed value of p<0.05 was considered to be significant.
A total of 67 patients were diagnosed with PTs during the study period with ages ranging from 14 to 61 yr (mean 37.6±11.3 yr). Fifty-nine patients underwent lumpectomy/excisional biopsy, while three underwent vacuum-assisted excision and another five had total mastectomies. Tumor size ranged from 1.2 to 12.0 cm (mean 5.0±2.5 cm). Histologic classification revealed 39 (58.2%) benign, 16 (23.9%) borderline, and 12 (17.9%) malignant PTs. Forty-six tumors (68.7%) were well circumscribed, but 21 patients (31.3%) had infiltrative tumor borders. Four patients (2 benign, 1 borderline, 1 malignant) had positive surgical margins, and 17 were close to the margin (within 0.2 cm of the margin) (9 benign, 3 borderline, 5 malignant) (Table 3, Figure 1). Of the 67 PTs, 11 cases (16.4%) recurred from 3 to 92 months following initial diagnosis (4 benign, 2 borderline, 5 malignant). Among 11 patients who experienced recurrent disease, nine (3 benign, 2 borderline, and 4 malignant) had positive or close surgical margins at original resection (Table 3). One benign PT case recurred as a borderline tumor. Further, two borderline PT cases recurred as malignancies (Table 4, Figure 2), five patients suffered from metastasis to the lungs or mesentery, and three patients died of malignant PT (Table 4).
Table 3 shows the overall rate of positivity among benign, borderline, and malignant PTs. p53 stromal cell immunopositivity was observed in 10.3% of benign PTs, 31.3% of borderline PTs, and 50.0% of malignant PTs. The Ki-67 index increased in 5.1% of benign PTs, 25.0% of borderline PTs, and 75.0% of malignant PTs. c-Kit stromal cell immunopositivity was observed in 7.7% of benign PTs, 31.3% of borderline PTs, and 66.7% of malignant PTs. p16 stromal cell immunopositivity was observed in 12.8% of benign PTs, 37.5% of borderline PTs, and 75.0% of malignant PTs. PDGFRA stromal cell immunopositivity was observed in 30.8% of benign PTs, 43.8% of borderline PTs, and 83.3% of malignant PTs (Figure 3).
Single-stranded conformational polymorphism-polymerase chain reaction (SSCP-PCR) analysis for mutation in exons 9, 11, 13, and 17 of the c-Kit gene, and exons 12 and 18 of PDGFRA was carried out in 16 borderline tumors and 12 malignant tumors. No mutations of the c-Kit or PDGFRA genes were found.
There was no statistically significant association of tumor borders (infiltrative vs. pushing), surgical method (mastectomy vs. excisional biopsy), either p53 or p16 immunostaining with recurrent disease (p>0.05). However, margin status (p=0.000), Ki-67 (p=0.012), c-Kit (p=0.002), and PDGFRA (p=0.007) stromal immunopositivity were significantly correlated with recurrence (Table 5, Figure 4). Multivariate analytical results revealed that only PDGFRA (p=0.040) immunopositivity, among immunohistochemical parameters, was correlated with recurrence (Table 6).
The majority of PTs are benign and patient risk focuses on local recurrence rather than metastatic disease. The most powerful predictor of local recurrence is the completeness of excision.(1) Local recurrence occurs in both benign and malignant tumours(6) with a approximately 15-20% of cases developing recurrence,(1) the majority of whom do so within 2-3 yr after initial diagnosis.(19) In this series, 11 of 67 PT cases (16.4%) showed recurrence between three and 92 months after initial diagnosis. Among 11 recurrent cases, four were benign, two borderline, and five malignant.
Subsequent recurrence may show increased cellularity, significant nuclear atypia, and increased mitotic activity.(19) Recurrence may mirror the microscopic pattern of the original tumor or show dedifferentiation.(20) According to Grimes,(20) mitotic rates in recurrences increased compared with the primary tumor in 10 of 14 benign tumors and five of seven borderline tumors. In five of these benign tumors, the recurrent tumors were histologically malignant and in four borderline tumors, the recurrences were classified as histologically malignant.(20) In this study, two borderline PT cases recurred as malignancies and one benign PT case recurred as a borderline tumor.
According to Kleer et al.,(21) three of seven (42.9%) benign PTs recurred locally, two of which had close margins (within 0.1 cm of the margin), and one had positive margins (tumor at the inked margin). Two of these three tumors recurred as histologically benign PT and one as high-grade malignant PT.(21) Among the four benign PTs which recurred in our study, two cases showed a positive margin and one case had less than 0.2 cm clear surgical margin. Further, two recurred borderline and 4 malignant tumors had positive or close surgical margins at original resection. The importance of adequate margins in the treatment of benign and malignant PTs should be emphasized. A one centimeter margin of surrounding benign tissue is recommended, even though this is not routine in all centers.(1,21)
The reported rate of metastasis and death due to malignant PTs has varied from 0% to 40%. The likelihood of metastatic disease has been, to some extent, related to stromal overgrowth and cellularity, as well as cytologic atypia and mitotic activity and, in some series, to the presence of necrosis.(1) In another report, recurrence was observed in 6 of 23 malignant PT cases (26.1%) and the 5-yr disease free survival rate was 48.9%. Frequent mitosis (>10/10HPF) and an invasive margin were the principal determinants of recurrence.(22) In this series, four patients (6.0%) suffered from metastasis to the lungs or mesentery and three (4.5%) died of metastatic malignant PTs.
A variety of immunohistochemical markers, including MIB1 monoclonal antibody to cell proliferation-associated Ki-67 antigen, p53, VEGF, CD10, c-Kit, and PDGFRA have been used in an attempt to predict behavior and more accurately classify PTs.(1,7,8,10,16,23) Both Ki-67 and p53 expression correlate with the histologic classification of PT.(21,24-26) However, neither Ki-67 nor p53 expression has been correlated with clinical behavior.(21) It has been suggested that malignant PTs have diffuse strong staining for p53 allowing for the distinction of malignant from benign and borderline PTs; however, p53 protein expression has not been able to predict outcome.(26,27) Negative or weak staining for the p53 protein in PT is of little discriminatory value.(27)
Expression of the CD117 protein within stromal cells has been significantly associated with a myriad of morphologic parameters, including grade and recurrent disease.(10) Further, the stromal expression of c-Kit has been reported to be increased in malignant PTs compared to benign PTs.(7,8,16) However, Tse et al.(9) reported that c-Kit was not related to recurrence or metastasis. Additionally, in a study by Esposito et al.,(11) c-Kit was differentially expressed among tumor grades, but the difference in its expression between borderline and malignant PTs was not significant, and c-Kit expression did not significantly correlate with tumor recurrence. Thus, the significance of c-Kit expression in PTs is uncertain. According to Tan et al.,(10) 13% of PT patients recurred during 30.3 mo of the mean follow-up period. CD117 stromal immunopositivity was correlated with recurrence (p=0.001); however, there was no association with p53 immunostaining. Activating mutations of the c-Kit gene similar to those described for GISTs appear to be rare or absent in malignant PTs.(7,8,16)
Carvalho et al.(16) reported c-Kit expression in 12 of 19 cases (6 of 13 benign cases and all 6 malignant ones) and PDGFFRA expression in 2 of 19 cases (one of 6 malignant PT cases and one of 13 benign cases). In our study, PDGFRA immunopositivity was higher than these reported results (33.3% of benign PTs, 43.8% of borderline PTs, and 83.3% of malignant PTs). In this study, >10% immunoreactivity was considered to be positive. However, in the Carvalho et al.(16) study, they interpreted >25% c-Kit and PDGFRA immunoreactivity to be positive and found that the 2415 c>T alteration in exon 17 of the c-Kit gene was present in two benign PTs. Further, analysis of exons 12 and 18 of the PDGFRA gene showed an intronic insertion, IVS17-50T, and an exonic silent alteration, 2866G>T, in exon 18. However, these alterations have also been found in the normal population, suggesting that they could be population polymorphisms.(16) In this series, no mutations of the c-Kit or PDGFRA genes were found. Recently, Paulsson et al.(28) reported that high stromal PDGF beta-receptor expression in breast cancer was significantly associated with high histopathological grade, estrogen receptor negativity, and high HER2 expression. These findings highlight the prognostic significance of stromal markers and should be considered in the ongoing clinical development of PDGF receptor inhibitors.
Even though positive or close margins have been significantly associated with tumor recurrence, we have shown that stromal c-Kit and PDGFRA positivity, and the Ki-67 index are useful for predicting the recurrence of PTs. Further, no mutations of c-Kit or PDGFRA were found in the current study. The underlying molecular mechanism of PT progression remains poorly understood and therefore, further molecular studies are warranted.
Figures and Tables
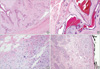 | Figure 1A well circumscribed, pushing border of a benign phyllodes tumor (A and B, H&E stain, ×40). An infiltrative border (C, H&E stain, ×400) and a positive surgical margin of a malignant phyllodes tumor (D, H&E stain, ×40). |
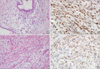 | Figure 2Borderline phyllodes tumor (A, H&E stain, ×200) with a high c-Kit positivity (B, immunohistochemical stain, ×400) recurred as a malignant phyllodes tumor (C, immunohistochemical stain, ×200) with stromal overgrowth and platelet derived growth factor receptor alpha positivity (D, immunohistochemical stain, ×400). |
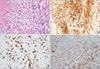 | Figure 3Malignant phyllodes tumor (A, H&E immunohistochemical stain, ×400) showing c-kit positivity (B, immunohistochemical stain, ×400), a high Ki-67 index (C, immunohistochemical stain, ×400), and platelet derived growth factor receptor alpha positivity (D, immunohistochemical stain, ×400) in stromal cells. |
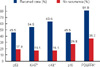 | Figure 4Immunohistochemical staining results for the recurrence of phyllodes tumors and phyllodes tumors with no recurrence (*p<0.05).
PDGFRA=platelet derived growth factor receptor alpha.
|
References
1. O'Malley F, Pinder SE. Breast Pathology. 2006. Edinburgh: Churchill Livingstone Elsevier;118–124.
3. Jacklin RK, Ridgway PF, Ziprin P, Healy V, Hadjiminas D, Darzi A. Optimising preoperative diagnosis in phyllodes tumour of the breast. J Clin Pathol. 2006. 59:454–459.

5. Inoshita S. Phyllodes tumor (cystosarcoma phyllodes) of the breast. A clinicopathologic study of 45 cases. Acta Pathol Jpn. 1988. 38:21–33.

6. Bellocq JP, Margo G. Tavassoli FA, Devilee P, editors. Fibroepithelial tumours. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. 2003. Lyon: IARC Press;99–103.
7. Chen CM, Chen CJ, Chang CL, Shyu JS, Hsieh HF, Harn HJ. CD34, CD117, and actin expression in phyllodes tumor of the breast. J Surg Res. 2000. 94:84–91.

8. Sawyer EJ, Poulsom R, Hunt FT, Jeffery R, Elia G, Ellis IO, et al. Malignant phyllodes tumours show stromal overexpression of c-myc and c-kit. J Pathol. 2003. 200:59–64.

9. Tse GM, Putti TC, Lui PC, Lo AW, Scolyer RA, Law BK, et al. Increased c-kit (CD117) expression in malignant mammary phyllodes tumors. Mod Pathol. 2004. 17:827–831.

10. Tan PH, Jayabaskar T, Yip G, Tan Y, Hilmy M, Selvarajan S, et al. p53 and c-kit (CD117) protein expression as prognostic indicators in breast phyllodes tumors: a tissue microarray study. Mod Pathol. 2005. 18:1527–1534.

11. Esposito NN, Mohan D, Brufsky A, Lin Y, Kapali M, Dabbs DJ. Phyllodes tumor: a clinicopathologic and immunohistochemical study of 30 cases. Arch Pathol Lab Med. 2006. 130:1516–1521.

12. Carvalho I, Milanezi F, Martins A, Reis RM, Schmitt F. Overexpression of platelet-derived growth factor receptor alpha in breast cancer is associated with tumour progression. Breast Cancer Res. 2005. 7:R788–R795.
13. Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998. 11:728–734.
14. Miettinen M, Monihan JM, Sarlomo-Rikala M, Kovatich AJ, Carr NJ, Emory TS, et al. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999. 23:1109–1118.

15. Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000. 13:1134–1142.

16. Carvalho S, Silva AO, Milanezi F, Ricardo S, Leitão D, Amendoeira I, et al. c-KIT and PDGFRA in breast phyllodes tumours: overexpression without mutations? J Clin Pathol. 2004. 57:1075–1079.

17. Wardelmann E, Neidt I, Bierhoff E, Speidel N, Manegold C, Fischer HP, et al. c-kit mutations in gastrointestinal stromal tumors occur preferentially in the spindle rather than in the epithelioid cell variant. Mod Pathol. 2002. 15:125–136.

18. Yamamoto H, Oda Y, Kawaguchi K, Nakamura N, Takahira T, Tamiya S, et al. c-kit and PDGFRA mutations in extragastrointestinal stromal tumor (gastrointestinal stromal tumor of the soft tissue). Am J Surg Pahtol. 2004. 28:479–488.

19. Moinfar F. Essentials of Diagnostic Breast Pathology: A Practical Approach. 2007. Berlin: Springer-Verlag;321–323.
20. Grimes MM. Cystosarcoma phyllodes of the breast: histologic features, flow cytometric analysis, and clinical correlations. Mod Pathol. 1992. 5:232–239.
21. Kleer CG, Giordano TJ, Braun T. Pathologic, immunohistochemical, and molecular features of benign and malignant phyllodes tumors of the breast. Mod Pathol. 2001. 14:185–190.

22. Lee HS, Kim HA, Shin DS, Kim YH, Chung SY, Jin MS, et al. Risk factors for recurrence after surgical treatment of a malignant phyllodes tumor of the breast. J Breast Cancer. 2007. 10:248–253.

23. Song JY, Yoon HK. Immunohistochemical phenotypes of phyllodes tumor of the breast. Korean J Pathol. 2008. 42:151–156.
24. Kocová L, Skálová A, Fakan F, Rousarová M. Phyllodes tumour of the breast: immunohistochemical study of 37 tumours using MIB1 antibody. Pathol Res Pract. 1998. 194:97–104.

25. Millar EK, Beretov J, Marr P, Sarris M, Clarke RA, Kearsley JH, et al. Malignant phyllodes tumours of the breast display increased stromal p53 protein expression. Histopathology. 1999. 34:491–496.

26. Feakins RM, Mulcahy HE, Nickols CD, Wells CA. p53 expression in phyllodes tumours is associated with histological features of malignancy but does not predict outcome. Histopathology. 1999. 35:162–169.





 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download