Abstract
Purpose
This study was designed to investigate differences in ultrasonographic findings between malignant and benign mammary duct ectasia.
Methods
From January 2003 to June 2005, 54 surgically proven mammary duct ectasia lesions depicted on sonograms were included in this study. We evaluated the ultrasonographic (US) findings in terms of involved ductal location, size, margin, intraductal echogenicity, presence of an intraductal nodule, calcification, ductal wall thickening and echo changes of the surrounding breast parenchyma. The US findings were correlated with the pathological features.
Results
Of the 54 lesions, 46 lesions were benign and eight lesions were malignant. Benign lesions included an inflammatory change (n=7), ductal epithelial hyperplasia (n=7), fibrocystic change (n=18), intraductal papilloma (n=11), atypical ductal hyperplasia (n=2) and sclerosing adenosis (n=1). Malignant lesions included ductal carcinoma in situ (DCIS) (n=6), infiltrating ductal carcinoma (n=1) and mucinous carcinoma (n=1). On US images, the peripheral ductal location, an ill-defined margin, ductal wall thickening and a hypoechoic change of the surrounding parenchyma were features significantly associated with malignant duct ectasia.
Breast ultrasonography (US) is appropriate for the initial evaluation of women younger than 30 yr with a palpable lump and as an adjunctive method for the evaluation of mammographically detected masses, persistent focal asymmetric densities and palpable abnormalities not seen on mammography.(1) Recently, US has been widely used for screening in women with a dense breast parenchyma.
The analysis of US features of solid masses continues to improve, though observer variability remains to be problematic to avoid a biopsy.(2) The American College of Radiology illustrated Breast Imaging Reporting and Data System (BI-RADS) US lexicon is helpful to improve observer performance.(3)
BI-RADS defines ductal changes as an abnormal caliber and/or arborization and describes as a change of the surrounding tissue associated with a solid breast mass.(3) However, ductal changes, especially duct ectasia itself, is a frequently encountered finding during a US examination. Although several reports have described the galactographic findings of malignant duct ectasia,(4,5) US findings of duct ectasia have not yet been investigated and there is no morphological criteria suggesting malignant duct ectasia using the BI-RADS US lexicon. Thus, confusion remains in the description and management for this abnormality and radiologists often interpret findings in an arbitrary manner. In this study, we investigated differences of US features between malignant and benign mammary duct ectasia.
The institutional review board of Korea University Anam Hospital approved this study. This study was conducted for 54 sonographically detected mammary duct ectasia lesions in 51 female patients that were diagnosed pathologically from January 2003 to June 2005. We excluded cases that showed a definite solid mass on ultrasound. All were female and the mean patient age was 46 yr (age range, 32-63 yr). All 54 lesions were pathologically confirmed after a sonography-guided core biopsy using a 14-gauge needle (Bard, Covington, USA) and 11 lesions were surgically excised after a core biopsy.
A radiologist reviewed and recorded the clinical symptoms from the medical records. Breast US was performed using a broadband width (14-5 MHz) linear-array transducer with a LOGIQ 9 (GE Healthcare, Milwaukee, USA) or HDI 5000 unit (Philips Medical Systems, Bothell, USA). In our practices, entire breasts are scanned as a diagnostic workup or screening for women with dense breasts by one experienced breast radiologist and once an abnormal finding is detected, US is performed in the radial and antiradial planes as well as in the longitudinal and transverse planes.
Two experienced breast radiologists reviewed the US findings in consensus. The US findings of duct ectasia were evaluated in terms of lesion location, size, margin, intraductal echogenicity, presence of an intraductal nodule and calcification, ductal wall thickening and change of the surrounding parenchymal echo. The location of duct ectasia was divided into central (defined as less than 2 cm from the nipple) and peripheral locations (more than 2 cm from the nipple). The size of a duct ectasia lesion was measured by the longest length and divided into less than or greater than 1 cm. The margin of a lesion was described as well defined or ill defined and the intraductal echo pattern was described as homogeneous or heterogeneous. In addition, we investigated the presence of an intraductal nodule, intraductal calcification and ductal wall thickening. We also examined the echo change of the surrounding parenchyma. After that, based on our results, we gave one point to each suspicious sonographic findings and investigated the difference of malignancy rate according to the score. An experienced pathologist correlated the US findings with the histopathological features.
To compare the US findings of mammary duct ectasia between benign and malignant lesions, Fisher's exact test was used. Statistical analyses were performed by the statistician by the use of SPSS software (version 11.0; SPSS Inc., Chicago, USA). A p-value less than 0.05 was considered as statistically significant.
The clinical findings are shown in Table 1. Of the 54 cases, 24 cases (44%) were symptomatic and 30 cases (56%) were asymptomatic. The asymptomatic women were examined with US for cancer screening.
Although there was no statistical significance (p>0.05), malignant duct ectasia was more frequently symptomatic. Among the patients with malignancies, 63% of patients showed symptoms. Two patients had nipple discharge, three patients had a palpable mass and three patients underwent screening. In contrast, 41% of the patients with benign lesions were symptomatic.
Bloody nipple discharge was found for 10 cases (19%) including eight benign lesions and two malignant lesions. Among the eight benign lesions, seven lesions were papillomas that involved the central ducts. A palpable mass was seen for 14 cases (26%) that consisted of 11 benign lesions and three malignant lesions.
Of the 54 lesions, 46 lesions (85%) were benign and eight lesions (15%) were malignant based on the histology. The pathological diagnoses of 46 benign lesions included an inflammatory change (n=7), ductal epithelial hyperplasia (n=7), fibrocystic change (n=18), intraductal papilloma (n=11), atypical ductal hyperplasia (n=2) and sclerosing adenosis (n=1). The malignant lesions included ductal carcinoma in situ (DCIS) (n=6), infiltrating ductal carcinoma (n=1) and mucinous carcinoma (n=1) (Table 2).
US findings of mammary duct ectasia are summarized in Table 3. Of the 46 benign lesions, 74% (34/46) of the lesions developed from the central ducts (Figures 1, 2). In contrast, 88% (7/8) of the malignant lesions involved the peripheral ducts (p<0.05) (Figures 3, 5).
The mean sizes of the benign and malignant lesions were 1.5 cm and 1.8 cm, respectively. Of 46 benign lesions, 26 lesions (57%) were greater than 1 cm in length. Of eight malignant lesions, seven lesions (88%) were greater than 1 cm in length. This difference was not significant statistically (p>0.05).
Heterogeneous intraductal echo was more frequently seen for malignant lesions (88%) (Figures 3-5) as compared to benign lesions (63%). The presence of an intraductal nodule and calcification were found in 12% and 50% of malignant lesions, respectively (Figures 3, 4), and the presence of an intraductal nodule and calcification were found in 52% and 28% of benign lesions, respectively (Figures 1, 2). However, there was no significant difference in the lesion size, the pattern of intraductal echo and the presence of an intraductal nodule or calcification between benign and malignant duct ectasia lesions (p>0.05) (Table 3).
In terms of lesion margin, 88% (7/8) of malignant lesions demonstrated an ill-defined margin (Figures 3-5), but 85% (39/46) of benign lesions had a well-defined margin (Figures 1, 2) (p<0.05). Ductal wall thickening was seen for 75% (6/8) of malignant lesions (Figures 3-5), and for 13% (6/46) of benign lesions (p<0.05). Two of six benign lesions that showed ductal wall thickening were atypical ductal hyperplasia. Hypoechoic changes at the surrounding breast tissue were seen for 38% (3/8) of malignant lesions (Figure 5) and 4% (2/46) of benign lesions (p<0.05).
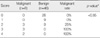
Table 4 presents the difference of malignancy rate by score. Based on our results, suspicious sonographic findings were peripheral location, ill defined margin, presence of ductal wall thickening and surrounding hypoechoic parenchymal changes. From this results, the malignancy rate of score 0 and 1 was 0%, of score 2 was 25%, and of score 3 and 4 was 100% (p<0.05).
Mammary duct ectasia is defined as a dilated duct larger than 2 mm in diameter or a dilated ampullary portion larger than 3 mm in diameter. Duct ectasia affects the major ducts in the subareolar region, but sometimes the smaller segmental ducts can be involved. Thick, unresorbed secretions and cellular debris may fill the distended ducts. Periductal fibrosis is found in association with an inflammatory infiltrates.(6) Based on our results, in contrast to benign lesions, most malignant duct ectasia lesions involved the peripheral ducts and the margin of duct ectasia lesions was ill-defined (p<0.05).
The cause of duct ectasia is not clear. Duct dilatation may be secondary to periductal inflammation where the ducts become patulous and the ducts are filled with unresorbed material and cellular debris.(6)
Reported diagnoses of pathological nipple discharge include intraductal papilloma (33-48%), fibrocystic disease (14-33%), papillomatosis (14-28%), mammary duct ectasia (2-11%) and a breast malignancy (8-15%).(7-10) In our study, 15% of mammary duct ectasia lesions were was malignant and three-quarters of the lesions were low grade DCIS. Cho et al.(11) reported that 17% of non-calcified DCIS lesions detected on US were manifested as pure ductal changes without a solid mass and all lesions were designated as group 1 based on the Van Nuys classification. The recent use of high resolution US can help to detect subtle findings of non-calcified DCIS, especially lesions that manifest as a pure ductal change.
The radiological findings of mammary duct ectasia based on mammography and galactography have been well described in many reports(4-6) but US features have not been investigated to the best of our knowledge.
On mammography,(6) duct ectasia appears as a tubular, serpinginous structure converging on the nipple at the subareolar region in the fatty breast. However, duct ectasia is not visible since the lesions are obscured by the dense breast parenchyma.
When a serous or bloody nipple discharge is present, ductography can be easily performed for the evaluation of the intraductal pathology. Several reports have described the ductographic findings according to the different pathological diagnoses.(12) Diner(12) showed that the ducts of fibrocystic disease may be irregular in diameter and may be displaced by cysts. lntraductal papilloma is depicted as a localized rounded or lobulated filling defect within the dilated major ducts in the subareolar region. For secretory disease, the ducts are markedly dilated, somewhat tortuous and may contain irregular filling defects produced by secretions. If the ducts are irregular in outline, encased and straightened, distorted or obstructed, the presence of an invasive carcinoma should be considered.(12)
A study by Hou et al.(4) demonstrated the galactographic findings of malignant tumors. Malignant duct ectasia is typically depicted as an irregular intraductal filling defect and ductal stenosis that affects the peripheral ducts. Cardenosa et al.(5) showed that a malignant intraductal nodule, pathologically proven DCIS or papilloid hyperplasia appear as irregular filling defects on galactography and benign lesions such as an intraductal papilloma are seen as a round well circumscribed filling defect involving the central ducts. Benign intraductal nodules are seen as a round well circumscribed intraductal filling defect, and a malignant intraductal nodule is more common in a peripheral duct and shows an irregular filling defect with the use of galactography.
When nipple discharge is vague or originates from multiple openings, successful ductography is impossible and the evaluation of intraductal pathology is difficult. In such cases, with US it is possible to visualize mammary duct ectasia distinctively regardless of nipple discharge and to evaluate the location, size and intraductal features such as the presence of a nodule or calcification and the echo-pattern. US is also able to evaluate a surrounding parenchymal echo change. Furthermore, US has an advantage of being a non-invasive procedure as compared to ductography. US should be performed in the radial and antiradial planes as well as in the longitudinal and transverse planes. Radial US is particularly useful to depict the intraductal pathology and to evaluate the extent of ductal disease, whereas antiradial US is more helpful to evaluate the surface characteristics of a mass.(2,13) However, US features suggestive of malignant duct ectasia have not yet been investigated.
Several histopathological conditions affect the intraductal echotexture. Calcified DCIS lesions usually show a mild hypoechoic, heterogeneous echotexture, probably due to the presence of punctuate echogenic calcifications.(13) Similar US findings may also be seen for some benign lesions such as sclerosing adenosis, atypical ductal hyperplasia and a radial scar.(14) Our study demonstrated heterogeneous intraductal hypoechogenicity in 88% (7/8) of malignant lesions and 63% (29/46) of benign lesions. Intraductal echogenicity was due to intraductal secretion, inflammatory cellular debris and intraductal calcification based on histological findings. In one case, intraductal heterogeneous hypoechogenicity was due to deposition of fibrin and lymphocytes within dilated ducts. However, in our study, the heterogeneity of intraductal echogenicity did not provide useful information to differentiate malignant from benign duct ectasia.
In our study, malignant duct ectasia was not associated with a discrete intraductal nodule except for one case. An intraductal nodule was commonly associated with benign duct ectasia, with intraductal papilloma as the most common finding.
Several studies have examined the clinical significance of microcalcifications detected on US.(15-17) Soo et al.(17) mentioned that suspicious microcalcifications were seen infrequently on sonography (23%), but when detected could be successfully biopsied with sonographic guidance and more frequently were malignant lesions and represented invasive cancer as compared to lesions seen on mammography alone. Likewise, in our study, 72% of benign lesions showed no intraductal calcifications but half of the malignant lesions contained intraductal calcification, even though this difference was not significant statistically. For cases that show suspicious sonographic features of duct ectasia, the presence of intraductal calcifications can be a helpful finding that can increase the confidence of a radiologist for a diagnosis of a malignancy.
From our results, the most striking feature of malignant duct ectasia was an ill-defined margin with ductal wall thickening. An ill-defined margin was seen for 88% of malignant lesions and 15% of benign lesions. Ductal wall thickening was seen only in 13% of benign lesions but in 75% of malignant lesions. Histological examinations revealed accumulation of tumor cells within ducts without evidence of invasion through the basement membrane for malignant lesions.
Pure clusters of microcysts without discrete solid components can be considered probably benign and should be subjected to follow-up. Such lesions are often due to apocrine metaplasia, though a fibrocystic change without apocrine metaplasia can have a similar appearance.(18) Berg(19) reported 66 lesions prospectively characterized as clustered microcysts with no malignancies. Our study showed a case that was depicted as several aggregated cysts with ill-defined duct ectasia and a surrounding hypoechoic parenchymal change. We considered the lesion as a probably benign lesion, but the lesion was diagnosed as a mucinous carcinoma with infiltrating ductal carcinoma component based on the histopathology. From our results, 38% (3/8) of malignant duct ectasia lesions and only 4% (2/46) of benign lesions demonstrated a hypoechoic change of the surrounding breast tissue. One malignant lesion was due to direct tumor cell infiltration but two cases were proliferative lesions without atypia and fibrocystic changes based on the histology.
In conclusion, mammary duct ectasia without a solid mass frequently depicted on breast US is a commonly benign condition, especially a fibrocystic change. In contrast, if there is peripheral, ill-defined duct ectasia with ductal wall thickening and associated hypoechogencity of the surrounding breast tissue, the possibility of a malignancy such as carcinoma in situ should be considered and radiologists should not hesitate to recommend a prompt biopsy.
Figures and Tables
 | Figure 1A 44-yr-old-woman without symptom and moderate to florid ductal epithelial hyperplasia. (A) Transverse (left) and longitudinal (right) scans of US reveal a well defined duct ectasia with intraductal homogeneous echogenecity (arrows) in the subareolar area of the left breast. An echogenic intraductal nodule (arrowhead) is shown with no evidence of intraductal calcification. (B) A photomicrograph demonstrates protruding ductal epithelial hyperplasia (arrow) and the hyperplastic epithelial gland, with moderate to florid ductal epithelial hyperplasia (arrowhead) (H&E stain, ×200). |
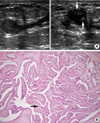 | Figure 2A 49-yr-old woman with left bloody nipple discharge and intraductal papilloma and ductal epithelial hyperplasia. (A) Radial (left) and antiradial scans (right) of the left central duct demonstrate an isoechoic intraductal nodule (arrows) within well defined duct ectasia. The intraductal echogenicity is homogeneous with no abnormal ductal wall thickening. (B) A papillary projected papilloma (arrow) is well depicted on a histological examination (H&E stain, ×200). |
 | Figure 3A 34-yr-old woman with a palpable lump in the right breast and comedo type DCIS. (A) Radial (left) and antiradial scans (right) of US show ill defined duct ectasia filled with intraductal heterogeneous echogenecities and intraductal nodules in the peripheral portion (5 cm apart from the nipple) of the right breast. There is an associated ductal wall thickening (arrows). (B) A photomicrograph demonstrates tumor cells that invade the epithelium and basement membrane (arrows) as well as central necrosis (H&E stain, ×200). |
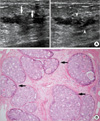 | Figure 4A 41-yr-old-woman with bloody nipple discharge from the left breast and cribriform DCIS. (A) Radial (left) and transverse (right) scans of US depict ill defined central duct ectasia with intraductal heterogeneous echogenicities. Associated intraductal nodules and calcifications (arrows) are well seen and irregular ductal wall thickening is prominent (arrowheads). (B) On a histological examination, tumor cells invade the ductal epithelium and basement membrane (arrows) (H&E stain, ×200). |
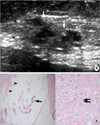 | Figure 5A 52-yr-old-woman with a palpable lump in the right breast and a mucinous carcinoma with infiltrating ductal components. (A) On US, several aggregated cysts with ill-defined duct ectasia are noted in the peripheral ducts (5 cm apart from the nipple) of the right breast. Intraductal heterogeneous hypoechogenicities with ductal wall thickenings (arrows) and a surrounding hypoechoic parenchymal change (arrowheads) are also demonstrated. (B) A photomicrograph demonstrates floating tumor cells (arrow) within the mucin pool (arrowheads) and associated infiltrating tumor cells (double arrows) (H&E stain, ×100). |
References
2. Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995. 196:123–134.

3. Mendelson EB, Berg WA, Merritt CR. Toward a standardized breast ultrasound lexicon, BI-RADS: ultrasound. Semin Roentgenol. 2001. 36:217–225.

4. Hou MF, Huang TJ, Liu GC. The diagnostic value of galactography in patients with nipple discharge. Clin Imaging. 2001. 25:75–81.

5. Cardenosa G, Eklund GW. Benign papillary neoplasms of the breast: mammographic findings. Radiology. 1991. 181:751–755.

6. Kopans DB. Kopans DB, editor. Histologic, pathologic, and image correlation. Breast Imaging. 2007. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;783–888.
8. Oh KK, Park YH, Ji H, Park CY. Values of contrast galactography in nonlactating nipple discharge in Korean women. J Korean Radiol Soc. 1988. 24:782–794.

9. Tabár L, Dean PB, Péntek Z. Galactography: the diagnostic procedure of choice for nipple discharge. Radiology. 1983. 149:31–38.

10. Cho N, Oh KK, Cho HY. Galactographic differentiation between malignant and benign disease in patients with pathologic nipple discharge. J Korean Radiol Soc. 2003. 48:511–516.

11. Cho KR, Seo BK, Kim CH, Whang KW, Kim YH, Kim BH, et al. Non-calcified ductal carcinoma in situ: ultrasound and mammographic findings correlated with histological findings. Yonsei Med J. 2008. 49:103–110.

13. Moon WK, Myung JS, Lee YJ, Park IA, Noh DY, Im JG. US of ductal carcinoma in situ. Radiographics. 2002. 22:269–280.

14. Stavros AT. Silverstein M, editor. Ultrasound of ductal carcinoma in situ. Ductal Carcinoma In Situ of the Breast. 1997. Baltimore: Williams & Wilkins;135–158.
15. Gufler H, Buitrago-Téllez CH, Madjar H, Allmann KH, Uhl M, Rohr-Reyes A. Ultrasound demonstration of mammographically detected microcalcifications. Acta Radiol. 2000. 41:217–221.

16. Cheung YC, Wan YL, Chen SC, Lui KW, Ng SH, Yeow KM, et al. Sonographic evaluation of mammographically detected microcalcifications without a mass prior to stereotactic core needle biopsy. J Clin Ultrasound. 2002. 30:323–331.

17. Soo MS, Baker JA, Rosen EL. Sonographic detection and sonographically guided biopsy of breast microcalcifications. AJR Am J Roentgenol. 2003. 180:941–948.

18. Warner JK, Kumar D, Berg WA. Apocrine metaplasia: mammographic and sonographic appearances. AJR Am J Roentgenol. 1998. 170:1375–1379.

19. Berg WA. Can clusters of microcysts appropriately be followed? 2002. Radiological Society of North America;abstract #E14-E510.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download