This article has been corrected. See "Erratum: Author's Name Correction" in Volume 13 on page 323.
Abstract
Purpose
Breast cancer is heterogeneous disease and the response to chemotherapeutic agents is also heterogeneous from patient to patient. Chemotherapy response assay is in vitro test that is performed to evaluate the degree of tumor growth inhibition by chemotherapy drugs. In this study, we performed the chemotherapy response assay using adenosine triphosphate (ATP-CRA) in breast cancer patients and assessed the clinical availability.
Methods
Sixty five breast cancer patients were enrolled in this study. Cancer cells were evenly divided and treated with commonly used chemotherapeutic drugs in breast cancer (doxorubicin, epirubicin, 5-fluorouracil, paclitaxel, docetaxel, vinorelbine, and gemcitabine). To verify in vitro ATP-CRA indirectly, we analyzed the correlation between cell death rate (CDR) of doxorubicin and epirubicin, and between doxorubicin and paclitaxel. We also analyzed the mean CDR of doxorubicin, epirubicin and paclitaxel by HER2 status.
Results
We could successfully perform the ATP-CRA in 60 patients (95.2%). In all cases, we can get the results within 7 days. The range of CDR was very wide, from 0 to more than 50%, except gemcitabine. Epirubicin showed the highest mean CDR (39.9%) and doxorubicin, paclitaxel in order. According to the chemosensitivity index, paclitaxel is the most frequently first-ranked and doxorubicin, epirubicin in order. Correlation coefficient between the cell death rate of doxorubicin and epirubicin is 0.4210 and 0.1299 between paclitaxel and doxorubicin. In HER2 positive group, mean CDR of paclitaxel, epirubicin and doxorubicin was higher than in HER2 negative group, even though epirubicin and doxorubicin were not statistically significant (p=0.018, p=0.114, p=0.311, respectively).
Conclusion
ATP-CRA showed heterogeneous results in individual patients. ATP-CRA was successful and can be performed within short time period. According to our in vitro study, it showed similar results with in vivo study but for the clinical use, the prospective randomized controlled trial should be preceded.
Figures and Tables
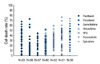 | Figure 1Cytotoxic effect aganist 7 anticancer drugs. A scatter gram shows heterogeneity of the chemosensitivity for anticancer drugs in the indicated number of patients with breast cancer. |
References
1. Eifel P, Axelson JA, Costa J, Crowley J, Curran WJ Jr, Deshler A, et al. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1-3, 2000. J Natl Cancer Inst. 2001. 93:979–989.

2. Dieras V, Fumoleau P, Romieu G, Tubiana-Hulin M, Namer M, Mauriac L, et al. Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. J Clin Oncol. 2004. 22:4958–4965.

3. Sjöström J, Krajewski S, Franssila K, Niskanen E, Wasenius VM, Nordling S, et al. A multivariate analysis of tumour biological factors predicting response to cytotoxic treatment in advanced breast cancer. Br J Cancer. 1998. 78:812–815.

4. Honkoop AH, Pinedo HM, De Jong JS, Verheul HM, Linn SC, Hoekman K, et al. Effects of chemotherapy on pathologic and biologic characteristics of locally advanced breast cancer. Am J Clin Pathol. 1997. 107:211–218.

5. Daidone MG, Veneroni S, Benini E, Tomasic G, Coradini D, Mastore M, et al. Biological markers as indicators of response to primary and adjuvant chemotherapy in breast cancer. Int J Cancer. 1999. 84:580–586.

6. Devita VT, Hellman S, Rosenberg SA. Cancer Principles & Practice of Oncology. 2001. 6th ed. Philadelphia: Lippincott Williams & Wilkins.
7. Cortazar P, Johnson BE. Review of the efficacy of individualized chemotherapy selected by in vitro drug sensitivity testing for patients with cancer. J Clin Oncol. 1999. 17:1625–1631.

8. Sevin BU, Peng ZL, Perras JP, Ganjei P, Penalver M, Averette HE. Application of an ATP-bioluminescence assay in human tumor chemosensitivity testing. Gynecol Oncol. 1988. 31:191–204.

9. Andreotti PE, Cree IA, Kurbacher CM, Hartmann DM, Linder D, Harel G, et al. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res. 1995. 55:5276–5282.
10. Myatt N, Cree IA, Kurbacher CM, Foss AJ, Hungerford JL, Plowman PN. The ex vivo chemosensitivity profile of choroidal melanoma. Anticancer Drugs. 1997. 8:756–762.

11. Cree IA, Neale MH, Myatt NE, de Takats PG, Hall P, Grant J, et al. Heterogeneity of chemosensitivity of metastatic cutaneous melanoma. Anticancer Drugs. 1999. 10:437–444.

12. Breidenbach M, Rein D, Schmidt T, Heindel W, Kolhagen H, Mallmann P, et al. Intra-arterial mitoxantrone and paclitaxel in a patient with Stewart-Treves syndrome: selection of chemotherapy by an ex vivo ATP-based chemosensitivity assay. Anticancer Drugs. 2000. 11:269–273.

13. Breidenbach M, Rein DT, Mallmann P, Kurbacher CM. Individualized long-term chemotherapy for recurrent ovarian cancer after failing high-dose treatment. Anticancer Drugs. 2002. 13:173–176.

14. O'Meara AT, Sevin BU. Predictive value of the ATP chemosensitivity assay in epithelial ovarian cancer. Gynecol Oncol. 2001. 83:334–342.
15. Cree IA, Kurbacher CM, Untch M, Sutherland LA, Hunter EM, Subedi AM, et al. Correlation of the clinical response to chemotherapy in breast cancer with ex vivo chemosensitivity. Anticancer Drugs. 1996. 7:630–635.

16. Kawamura H, Ikeda K, Takiyama I, Terashima M. The usefulness of the ATP assay with serum-free culture for chemosensitivity testing of gastrointestinal cancer. Eur J Cancer. 1997. 33:960–966.

17. Sharma S, Neale MH, Di Nicolantonio F, Knight LA, Whitehouse PA, Mercer SJ, et al. Outcome of ATP-based tumor chemosensitivity assay directed chemotherapy in heavily pre-treated recurrent ovarian carcinoma. BMC Cancer. 2003. 3:19.

18. Konecny G, Crohns C, Pegram M, Felber M, Lude S, Kurbacher C, et al. Correlation of drug response with the ATP tumorchemosensitivity assay in primary FIGO stage III ovarian cancer. Gynecol Oncol. 2000. 77:258–263.

19. Maehara Y, Anai H, Tamada R, Sugimachi K. The ATP assay is more sensitive than the succinate dehydrogenase inhibition test for predicting cell viability. Eur J Cancer Clin Oncol. 1987. 23:273–276.

20. Petty RD, Sutherland LA, Hunter EM, Cree IA. Comparison of MTT and ATP-based assays for the measurement of viable cell number. J Biolumin Chemilumin. 1995. 10:29–34.

21. Koechli OR, Avner BP, Sevin BU, Avner , Perras JP, Robinson DS, et al. Application of the adenosine triphosphate-cell viability assay in human breast cancer chemosensitivity testing: a report on the first results. J Surg Oncol. 1993. 54:119–125.

22. Ng TY, Ngan HY, Cheng DK, Wong LC. Clinical applicability of the ATP cell viability assay as a predictor of chemoresponse in platinum-resistant epithelial ovarian cancer using nonsurgical tumor cell samples. Gynecol Oncol. 2000. 76:405–408.

23. Haskell CM, Berek JS. Cancer Treatment. 2001. 5th ed. Philadelphia: W.B. Saunders.
24. Von Hoff DD, Clark GM, Stogdill BJ, Sarosdy MF, O'Brien MT, Casper JT, et al. Prospective clinical trial of a human tumor cloning system. Cancer Res. 1983. 43:1926–1931.
25. Von Hoff DD, Kronmal R, Salmon SE, Turner J, Green JB, Bonorris JS, et al. A Southwest Oncology Group study on the use of a human tumor cloning assay for predicting response in patients with ovarian cancer. Cancer. 1991. 67:20–27.

26. Shaw GL, Gazdar AF, Phelps R, Linnoila RI, Ihde DC, Johnson BE, et al. Individualized chemotherapy for patients with non-small cell lung cancer determined by prospective identification of neuroendocrine markers and in vitro drug sensitivity testing. Cancer Res. 1993. 53:5181–5187.
27. Shaw GL, Gazdar AF, Phelps R, Steinberg SM, Linnoila RI, Johnson BE, et al. Correlation of in vitro drug sensitivity testing results with response to chemotherapy and survival: comparison of non-small cell lung cancer and small cell lung cancer. J Cell Biochem Suppl. 1996. 24:173–185.

28. Wilbur DW, Camacho ES, Hilliard DA, Dill PL, Weisenthal LM. Chemotherapy of non-small cell lung carcinoma guided by an in vitro drug resistance assay measuring total tumour cell kill. Br J Cancer. 1992. 65:27–32.

29. Cortazar P, Gazdar AF, Woods E, Russell E, Steinberg SM, Williams J, et al. Survival of patients with limited-stage small cell lung cancer treated with individualized chemotherapy selected by in vitro drug sensitivity testing. Clin Cancer Res. 1997. 3:741–747.
30. Xu JM, Song ST, Tang ZM, Jiang ZF, Liu XQ, Zhou L, et al. Predictive chemotherapy of advanced breast cancer directed by MTT assay in vitro. Breast Cancer Res Treat. 1999. 53:77–85.

31. Kurbacher CM, Cree IA, Bruckner HW, Brenne U, Kurbacher JA, Müller K, et al. Use of an ex vivo ATP luminescence assay to direct chemotherapy for recurrent ovarian cancer. Anticancer Drugs. 1998. 9:51–57.





 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download