Abstract
Purpose
A better predictive model for occult invasive disease in ductal carcinoma in situ (DCIS) patients is essential to guide the tailored use of sentinel node biopsies. We hypothesized that recent improvement of contrast-enhanced breast magnetic resonance imaging (MRI) could provide more accurate information on the presence of occult invasion in DCIS patients.
Methods
From a prospectively maintained database, we identified 143 DCIS patients diagnosed with needle biopsies in whom MRI images were available.
Results
Sixty-five patients (45.5%) were upstaged to invasive carcinoma after curative surgery. Ultrasonographic lesion size, mass-appearance on mammography, type of needle used, and the presence of suspicious microinvasive foci were associated with increased likelihood of upstaging. Among the features of MRI, only mass-appearance was significantly associated with the presence of invasive disease (p=0.002). However, up to 50% of masses in MRI cases had massappearance on mammography as well. Other morphologic and pharmacokinetic features of MRI, such as shape, margin, and patterns of enhancement and washout, did not have a significant association.
Conclusion
Among various morphologic and pharmacokinetic parameters of contrast-enhanced MRI, only mass-appearance was associated with occult invasive disease. Our results show the limitations of current contrast-enhanced MRI in predicting invasive disease in patients with preoperative diagnoses of DCIS.
Although recent understanding of tumor biology has lead to improved management of ductal carcinoma in situ (DCIS), there are still certain areas that are controversial.(1) Among them lies the issue of performing sentinel node biopsy in patients with a preoperative diagnosis of DCIS.(2) The underlying rationale for performing sentinel node biopsy in DCIS patients is the possible upstaging to invasive cancer, which happens in up to 50% of needle biopsy cases,(3,4) a misfortune that almost always leads to additional surgical procedures for axillary staging if a sentinel node biopsy has not been performed at the time of initial surgery. A recent meta-analysis suggested that the incidence of a positive sentinel node in the preoperative diagnosis of DCIS is 7.4% based on 22 published studies, and concluded in favor of the routine use of the procedure.(5)
However, current guidelines on DCIS management recommend against the routine use of sentinel node biopsy, especially if the patient is suitable for breast conservation.(6,7) These guidelines are mainly the result of consensus among those who acknowledge both the low yield rate of a positive sentinel node in patients with localized DCIS and the potential long-term sequelae that may accompany the procedure.(8,9) Despite these recommendations, a recent review of the Surveillance, Epidemiology and End Results (SEER) data from 1998 to 2002 revealed that 22.5% of DCIS patients undergoing breast conservation had received axillary lymph node assessment, and 67% of the assessments were axillary dissections.(10) These apparent discrepancies between recommendations and clinical practice reflect the lack of a validated model to predict a subset of patients with high risk of invasive cancer, which would enable more tailored patient selection.
Recent researches involving the use of magnetic resonance imaging (MRI) in DCIS patients had shown an increased detection rate and more accurate estimation of tumor extent with MRI when compared to conventional mammography.(3,11-14) Furthermore, studies have suggested that the pharmacokinetics of contrast-enhanced MRI (patterns of enhancement and washout) may provide more detailed information regarding the presence of occult invasion in DCIS patients.(15,16) Other imaging modalities, such as ultrasonography, have shown limitations in predicting invasive disease.(4) In this study, we hypothesized that morphologic and pharmacokinetic features of contrast-enhanced MRI may predict the presence of occult invasion in patients with preoperative diagnosis of DCIS, and will thereby guide the selective use of sentinel node biopsy in these patients.
This was a retrospective study based on a prospectively maintained database. The database includes clinicopathologic and follow-up data of all patients who underwent breast surgery at the Seoul National University Hospital. Patients with a preoperative diagnosis of DCIS who underwent surgery in the Seoul National University Hospital between January 2007 and July 2008 were identified from a prospectively maintained database in the Seoul National University Hospital Breast Care Center.(17) During the study period, a total of 180 consecutive patients with a preoperative diagnosis of DCIS underwent curative surgery. Among them, 11 patients who received prior surgical excision of the suspicious lesion were excluded from analysis leaving 169 patients diagnosed by 11-gauge vacuum assisted or 14 gauge automated needle biopsies. Preoperative contrast-enhanced MRIs were not done in 26 patients for various reasons (e.g., patients'refusal, renal dysfunction). The remaining 143 patients were included in the analysis. Each patient underwent contrast-enhanced MRI before surgery to determine the extent of the tumor and the presence of occult multifocal lesions. Our protocols for MRI image acquisition and pathologic examination of breast cancer patients have been previously described.(17) Briefly, MRI was carried out with a 1.5-T imager (Sonata; Siemens Medical Systems, Erlangen, Germany or Signa; General Electric Medical Systems, Milwaukee, USA) using a dedicated breast coil (Liberty 5000; USA Instruments, Aurora, USA). A bolus of gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany) was injected i.v. at a dose of 0.1 mmol/kg of body weight within 15 s, followed by a 20 mL saline solution flush. MRI images were reviewed by a specialized radiologist who was blinded to the pathologic information of the studied patients. Morphologic and kinetic features of MRI images were described according to the BIRADS (Breast Imaging Reporting and Data System) MRI lexicon.(18)
Needle biopsies were performed with an 11-gauge vacuum-assisted device (Mammotome; Ethicon-Endosurgery, Cincinnati, USA) or a 14-gauge automated gun (Pro-Mag 2.2; Manan Medical Products, Northbrook, USA) under sonographic guidance with 10- or 12-MHz linear transducers (Kretz-Medicon; Seoul, Korea; HDI 5000; Advanced Technology Laboratories, Bothel, USA). For lesions not visible on ultrasonography, a wire was localized under mammographic guidance and 11-gauge vacuum-assisted biopsies were done under sonographic monitoring of the inserted wires. Each biopsy specimen was stained with hematoxylin and eosin and examined by a specialized breast pathologist.
Statistical analysis was done using a two-tailed chi-square test and Student's t-test to compare differences in nominal and continuous variables between groups, respectively. The SPSS for Windows statistical software package (SPSS Inc., Chicago, USA) was used to do the analyses. A p value of <0.05 was considered statistically significant.
Demographic and pathologic information of the studied patients are shown in Table 1. Preoperative diagnoses were made by 11-gauge vacuum assisted biopsy in 64 patients (44.8%) and by 14-gaude needle biopsy in 79 patients (55.2%). Among the 143 patients in whom the preoperative diagnoses were DCIS, 65 patients (45.5%) were upstaged to invasive carcinoma after curative resection. Contrast-enhanced MRI predicted tumor size more accurately than ultrasonography did (Pearson's correlation coefficients of 0.665 and 0.556, respectively).
The association of occult invasion and various clinicopathologic parameters was examined and is shown in Table 2. In accordance with our previous report,(4) the presence of a physically palpable lesion, a mass on mammography, and the ultrasonographic lesion size were significantly associated with the presence of invasive cancer. Additionally, patients who were diagnosed by 14-gauge needle biopsy and in whom initial pathologic findings showed suspicious microinvasion had a significantly higher incidence of occult invasive cancer (OR of 2.57 [95% CI, 1.29-5.11] and 3.59 [1.38-9.32], respectively). A higher histologic grade and the presence of comedo necrosis did not show a significant relationship with occult invasion.
In terms of morphologic parameters of contrast-enhanced MRI and their association with occult invasion, only mass-appearance on MRI was significantly associated with the presence of invasive cancer (OR of 3.56 [95% CI, 1.58-8.04], p=0.002). However, among the 35 patients who had mass-appearance in MRI, 17 patients (48.6%) had mass-appearance in mammography as well. Other morphologic parameters, such as margin, shape, and enhancement pattern, did not have a significant association with the presence of invasive cancer (Table 3). Furthermore, MRI pharmacokinetic characteristics of the tumor (initial enhancement and washout pattern) did not predict occult invasive cancer (Table 3). Subgroup analysis according to the size of needle (14 gauge vs. 11 gauge) also showed a lack of significant association between certain MRI characteristics and the presence of invasive cancer.
Our hypothesis that contrast-enhanced MRI characteristics may predict the presence of occult invasion in patients with a preoperative diagnosis of DCIS was mainly based upon two aspects. The first was the recent interest in the use of MRI in breast cancer patients.(14) Contrast-enhanced MRI provides more detailed and accurate information in certain breast cancer patients when compared to conventional mammography and ultrasonography. although its role in DCIS in still controversial.(19) The other motivation came from the underlying mechanism of contrast-enhanced MRI in delineating malignant breast tumors, which is the leak of contrast agent into the highly vascularized tissues located in and around the breast cancer cell nests. Recent studies have shown that molecular changes in stromal tissue of pure in situ breast tumors play a critical role in tumor invasion and progression.(20) Furthermore, vascular stroma formation or neo-vascularization may precede the invasion of in situ tumors.(21,22) Based on these recent observations, we postulated that stromal changes in DCIS patients may enable the contrast-enhanced MRI to select high risk patient group of invasive disease.
However, in our study, we could not identify contrast-enhanced MRI characteristics that predict the presence of occult invasion except for mass appearance on MRI. Previous studies have shown that DCIS patients with a clinically palpable breast mass or a radiographic breast nodule have increased risk of having occult invasive disease.(4,23-25) Our finding of an association between mass appearance on MRI and occult invasive cancer is in concordance with these previous reports. However, even among patients with mass appearance on MRI, about 30% had no evidence of invasive carcinoma and up to 50% also showed mass-appearance in mammography, reflecting the limitation of conventional contrast-enhanced breast MRI and the need for a more accurate prediction model.
Hwang et al.(16) in their study of 51 DCIS patients showed improved sensitivity and negative predictive value in predicting occult invasion in DCIS patients by using contrast-enhanced MRI. Additionally, from their analysis of 15 DCIS patients Groves et al.(15) reported the association of certain enhancement patterns and MRI shapes with pure DCIS. Certain morphologic and pharmacokinetic features of contrast-enhanced MRI such as a spiculated margin and a rapid washout pattern have often been suggested as predictive features of invasive disease.(16) However, in our study, mass shape, margin, enhancement pattern, and pharmacokinetic features of contrast-enhanced MRI did not have significant predictive value. Similarly, Facius et al.(26) showed, in their study of 74 patients, that 68% of pure DCIS had MRI features of invasive breast cancer.
Although we could not demonstrate highly accurate MRI features that predict occult invasion in DCIS patients, we believe that our results should not discourage the efforts of others in developing novel imaging biomarkers in DCIS patients. For example, recent advances in diffusion-weighted MRI and other molecular imaging could lead to a more tailored approach in DCIS patients.(1) Meanwhile, however, efforts should be made to improve and develop a novel MRI lexicon that may predict occult invasion and thereby guide the use of sentinel node biopsy in DCIS patients. Another important direction in developing a valid predictive model to provide a more tailored use of sentinel node biopsy in DCIS patients would be the molecular approach. Recent research advances have given novel insights into the progression and invasion process of DCIS in terms of molecular expression profiles and stromal phenotypes.(20,27-30) Potential molecular markers from recent studies should be validated in a prospective setting to determine their possible role as molecular markers for selecting DCIS candidates for sentinel node biopsy.
In this study of 143 consecutive patients, we tried to demonstrate the clinical usefulness of contrast-enhanced MRI in predicting invasive disease for patients with preoperative diagnoses of DCIS. Among the features of MRI, only mass-appearance was significantly associated with the presence of invasive disease. However, about 50% instances of mass on MRI cases had mass-appearance on mammography as well. Other morphologic and pharmacokinetic features of MRI, such as shape, margin, and patterns of enhancement and washout, did not have a significant association. Our results show the limitations of current contrast-enhanced MRI in predicting invasive disease in patients with preoperative diagnoses of DCIS.
Figures and Tables
Table 2
Association between various clinicopathologic factors and the presence of occult invasion
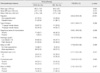
DCIS=ductal carcinoma in situ; IDC=invasive ductal carcinoma; OR=odds ratio; CI=confidence interval; SD=standard deviation; MRI=magnetic resonance imaging; USG=ultrasonography; MMG=mammography.
*Pathologic information regarding histologic grades was not available for 10 patients; †Ultrasonographic lesion size was unmeasurable in 20 patients; ‡Pathologic information regarding the presence of comedonecrosis was not available in 3 patients.
References
1. Kuerer HM, Albarracin CT, Yang WT, Cardiff RD, Brewster AM, Symmans WF, et al. Ductal carcinoma in situ: state of the science and roadmap to advance the field. J Clin Oncol. 2009. 27:279–288.

3. Sakorafas GH, Farley DR, Peros G. Recent advances and current controversies in the management of DCIS of the breast. Cancer Treat Rev. 2008. 34:483–497.

4. Lee JW, Han W, Ko E, Cho J, Kim EK, Jung SY, et al. Sonographic lesion size of ductal carcinoma in situ as a preoperative predictor for the presence of an invasive focus. J Surg Oncol. 2008. 98:15–20.

5. Ansari B, Ogston SA, Purdie CA, Adamson DJ, Brown DC, Thompson AM. Meta-analysis of sentinel node biopsy in ductal carcinoma in situ of the breast. Br J Surg. 2008. 95:547–554.

6. Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005. 23:7703–7720.

7. Morrow M, Strom EA, Bassett LW, Dershaw DD, Fowble B, Giuliano A, et al. Standard for breast conservation therapy in the management of invasive breast carcinoma. CA Cancer J Clin. 2002. 52:277–300.

8. Leonard GD, Swain SM. Ductal carcinoma in situ, complexities and challenges. J Natl Cancer Inst. 2004. 96:906–920.

9. Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006. 98:599–609.

10. Porembka MR, Abraham RL, Sefko JA, Deshpande AD, Jeffe DB, Margenthaler JA. Factors associated with lymph node assessment in ductal carcinoma in situ: analysis of 1988-2002 seer data. Ann Surg Oncol. 2008. 15:2709–2719.

11. Kuhl CK, Schrading S, Bieling HB, Wardelmann E, Leutner CC, Koenig R, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007. 370:485–492.

12. Schouten van der Velden AP, Boetes C, Bult P, Wobbes T. The value of magnetic resonance imaging in diagnosis and size assessment of in situ and small invasive breast carcinoma. Am J Surg. 2006. 192:172–178.

13. Hata T, Takahashi H, Watanabe K, Takahashi M, Taguchi K, Itoh T, et al. Magnetic resonance imaging for preoperative evaluation of breast cancer: a comparative study with mammography and ultrasonography. J Am Coll Surg. 2004. 198:190–197.

14. Hylton N. Magnetic resonance imaging of the breast: opportunities to improve breast cancer management. J Clin Oncol. 2005. 23:1678–1684.

15. Groves AM, Warren RM, Godward S, Rajan PS. Characterization of pure high-grade DCIS on magnetic resonance imaging using the evolving breast MR lexicon terminology: can it be differentiated from pure invasive disease? Magn Reson Imaging. 2005. 23:733–738.

16. Hwang ES, Kinkel K, Esserman LJ, Lu Y, Weidner N, Hylton NM. Magnetic resonance imaging in patients diagnosed with ductal carcinoma-in-situ: value in the diagnosis of residual disease, occult invasion, and multicentricity. Ann Surg Oncol. 2003. 10:381–388.

17. Moon HG, Han W, Lee JW, Ko E, Kim EK, Yu JH, et al. Age and HER2 expression status affect MRI accuracy in predicting residual tumor extent after neo-adjuvant systemic treatment. Ann Oncol. 2009. 20:636–641.

18. American College of Radiology. ACR BI-RADS Breast Imaging Reporting and Data System: Breast Imaging Atlas. 2003. 4th ed. Reston: American College of Radiology.
19. Orel S. Who should have breast magnetic resonance imaging evaluation? J Clin Oncol. 2008. 26:703–711.

20. Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008. 13:394–406.

21. Brown LF, Guidi AJ, Schnitt SJ, Van De Water L, Iruela-Arispe ML, Yeo TK, et al. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res. 1999. 5:1041–1056.
22. Gadre SA, Perkins GH, Sahin AA, Sneige N, Deavers MT, Middleton LP. Neovascularization in mucinous ductal carcinoma in situ suggests an alternative pathway for invasion. Histopathology. 2008. 53:545–553.

23. Goyal A, Douglas-Jones A, Monypenny I, Sweetland H, Stevens G, Mansel RE. Is there a role of sentinel lymph node biopsy in ductal carcinoma in situ?: analysis of 587 cases. Breast Cancer Res Treat. 2006. 98:311–314.

24. Jackman RJ, Burbank F, Parker SH, Evans WP 3rd, Lechner MC, Richardson TR, et al. Stereotactic breast biopsy of nonpalpable lesions: determinants of ductal carcinoma in situ underestimation rates. Radiology. 2001. 218:497–502.

25. Wilkie C, White L, Dupont E, Cantor A, Cox CE. An update of sentinel lymph node mapping in patients with ductal carcinoma in situ. Am J Surg. 2005. 190:563–566.

26. Facius M, Renz DM, Neubauer H, Bottcher J, Gajda M, Camara O, et al. Characteristics of ductal carcinoma in situ in magnetic resonance imaging. Clin Imaging. 2007. 31:394–400.

27. Nielsen BS, Rank F, Illemann M, Lund LR, Dano K. Stromal cells associated with early invasive foci in human mammary ductal carcinoma in situ coexpress urokinase and urokinase receptor. Int J Cancer. 2007. 120:2086–2095.

28. Hu M, Peluffo G, Chen H, Gelman R, Schnitt S, Polyak K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci U S A. 2009. 106:3372–3377.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download