Abstract
Purpose
Our purpose was to identify the factors in a breast core needle biopsy (CNB) of atypical ductal hyperplasia (ADH) that are predictive for carcinoma in the subsequent excision specimens.
Methods
We performed a retrospective pathologic review of 50 cases that were diagnosed as ADH via the CNB and that had the corresponding excision specimens.
Results
The size of the largest ADH foci in the CNBs was 0.8±0.6 mm (mean±SD) for benign proliferative disease (BPD, n=12), 1.0±0.5 mm (mean±SD) for ADH (n=9) and 1.3±0.8 mm (mean±SD) for malignant lesions (n=29) in excision specimens (p=0.105). Among the 30 cases showing stromal alterations around the ducts with ADH in the CNBs, 9 cases (30.0%) were BPD or ADH and 21 cases (70.0%) were malignant lesions in the excision specimens (p=0.004).
Breast core needle biopsy (CNB) is an established method for diagnosing benign and malignant breast disease and CNB is widely used in daily practice. However, CNB samples only part of a lesion, and this can be the reason for an inaccurate diagnosis.(1) This size limitation of CNB gives rise to important diagnostic problems because one of the important factors for making the differential diagnosis between atypical ductal hyperplasia (ADH) and low-grade ductal carcinoma in situ (DCIS) is the size of the lesion.(2,3) Previous studies have reported that ADH in a CNB showed significant discordance in the subsequent surgical excision specimens for which 33-87% demonstrated ductal carcinoma such as DCIS and invasive ductal carcinoma (IDC).(4-11) Therefore, surgical excision is presently recommended when ADH is diagnosed in a CNB. Investigations to determine the factors that are predictive of more advanced lesions in surgical excision specimens when ADH was diagnosed in the CNB have suggested cytologic atypia,(11) more than 4 foci (7) and micropapillary architecture (7), yet consistent results were not demonstrated.
The purpose of this study was to identify the predictive factors that suggest the diagnosis of ductal carcinoma in the subsequent surgical excision specimens when ADH is diagnosed in a CNB.
The surgical pathology archives at Severance Hospital were searched for those breast CNBs that were diagnosed as ADH and there were subsequent surgical excision specimens between January 2000 and December 2008. This study was approved by the Institutional Review Board of Severance University Hospital (4-2009-0285). Fifty cases with a main diagnosis of ADH alone were included. Sonographically guided core needle biopsies were performed, using the freehand technique, with a high-resolution sonographic unit and a 7.5- or 12-MHz linear array transducer (HDI 5000 or 3000, Philips ATL, Logic 9, GE Healthcare, Bothell, USA). All the biopsies were done as outpatient procedures under local anesthesia with the patient in the supine position. An automated gun (Pro-Mag 2.2; Manan Medical Products, Northbrook, USA) and a 14-gauge Tru-Cut needle with a 22-mm throw (SACN Biopsy Needle; Medical Device Technologies, Gainesville, USA) were used. The biopsies were performed by radiologists who specialized in interpreting breast images and performing percutaneous breast biopsies under sonographic guidance. Four or 5 core samples per lesion were obtained, according to our standard protocol. The breast CNBs were submitted for microscopic examination.
Microscopic examination of the CNB and the surgical excision tissue was performed retrospectively by a pathologist who specialized in breast pathology (JSK). The diagnostic criteria for ADH were those of Page and Rogers (3) with the size criteria of Tavassoli and Norris.(12) All the breast CNB slides were reviewed and the following data was recorded: the largest lesion size, the number of large ducts and/or terminal duct-lobular units affected, the architecture, stromal alterations and inflammation, and the number of biopsy cores. When the "ADH"-like changes were present in multiple cores, we measured the largest lesion size in any individual core. In addition, we measured the largest diameter of the ADH area by using the microscopic ruler contained in the microscope. The stromal alterations included fibroblast proliferation, fibrosis and sclerosis. When stromal alterations were evaluated, we compared the periductal stroma around the ADH to the periductal stroma around the coexisting normal ductal/lobular structures. A retrospective evaluation of slides from the subsequent surgical excision specimens was performed without the pathologist having any knowledge of the CNB findings. The surgical excision specimens were evaluated for the presence of DCIS, IDC and ADH. For the specimens showing carcinoma in the subsequent surgical excisions, the nuclear and/or histologic grade was recorded using the Van Nuys grading system (13) for DCIS and the Elston modification of the Scarff-Bloom-Richardson criteria (14) was used for IDC. The data was statistically processed using SPSS for Windows version 12.0 (SPSS Inc., Chicago, USA). For determining the statistical significance, Student's t test and Fisher's exact test were used for the continuous and categorical variables, respectively. Correlation analysis was performed by using Pearson's correlation test. Statistical significance was assumed for p values <0.05.
Table 1 shows the pathologic features of ADH in the CNBs. The size of the largest ADH focus was 1.1±0.7 mm (mean±SD). Among the 50 cases, the number of identifiable ADH foci was ≤2 in 23 cases (46.0%), 3-5 in 20 (40.0%) and ≥6 in 7 (14.0%). The architecture of the ADH was cribriform in 42 cases (84.0%), combined cribriform and micropapillary in 3 (6.0%), combined cribriform and papillary in 3 (6.0%), combined cribriform and solid in 1 (2.0%) and solid in 1 (2.0%). Stromal alterations such as fibrosis and fibroblast proliferation around the ductal structures showing ADH were noted in 30 (60.0%) cases (Figure 1) and the periductal stromal inflammation observed in 7 cases (14.0%) was predominantly composed of lymphocytes.
Table 2 demonstrates the final pathologic diagnosis of the subsequent surgical excision specimens. IDCs of 0.7 and 0.3 cm in size were identified in 2 cases (4.0%). In addition, DCIS components 1.2 and 2.0 cm in size were identified around the IDCs in these 2 cases. The nuclear and histologic grades of the IDCs in both cases were 1. DCIS was observed in 27 cases (54.0%). The architecture of the DCIS was cribriform in 16 cases (59.3%), combined cribriform and papillary in 8 (29.6%), combined cribriform and solid in 2 (7.4%) and papillary in 1 (3.7%). The Van Nuys grade was 1 (a non-high nuclear grade without comedonecrosis) in 16 cases (59.3%) and 2 (a non-high nuclear grade without comedonecrosis) in 11 cases (40.7%). The size of the DCIS was 1.3±0.6 cm (mean±SD; range, 0.3-3.0 cm). ADH was diagnosed in 9 cases (18.0%). The architecture was cribriform in all the ADH cases and the size of the ADH was 0.6±0.2 cm (mean±SD; range, 0.2-1.0 cm). Benign proliferative disease (BPD) without atypia was identified in 12 cases (24.0%); intraductal papilloma was identified in 5 (41.6%), fibrocystic disease was identified in 2 (16.6%), adenosis was identified in 1 (8.3%), complex sclerosing lesion was identified in 1 (8.3%), mucocele-like lesion was identified in 1 (8.3%), usual ductal hyperplasia was identified in 1 (8.3%) and lactational hyperplasia was identified in 1 (8.3%). Periductal fibrosis and sclerosis were noted in all 5 cases of intraductal papilloma.
Table 3 shows the correlation analysis between pathologic parameters in the CNBs and the pathologic diagnosis of the surgical excision specimens. The size of the largest ADH foci in the CNBs was 0.8±0.6 mm (mean±SD) for the final diagnosis of BPD, it was 1.0±0.5 mm (mean±SD) for the final diagnosis of ADH and it was 1.3±0.8 mm (mean±SD) for the final diagnosis of IDC or DCIS, without statistical significance (p=0.105). Stromal alterations around the ductal structures showing ADH in the CNB were noted in 8 cases (16.0%) of BPD, in 1 case (2.0%) of ADH and in 21 (42.0%) of IDC or DCIS with statistical significance (p=0.004). The number of ADH foci in the CNB, stromal inflammation, ADH architecture and the number of CNB cores showed no statistical significance between BPD, ADH and IDC/DCIS (p=0.315, 0.409, 0.685, and 0.585, respectively).
In this study, among the 50 patients who were diagnosed with ADH in their CNBs, 29 (58.0%) cases showed more advanced lesions such as DCIS and IDC in the surgical excision specimens. This figure is compatible with that of previous studies in which the underestimation rate of ADH in a CNB was 33-87%.(4-11) The wide range of underestimation was due to the differences in the ADH, the patient selection criteria and the number of biopsy cores. The major limitation of CNB is that it only investigates a restricted area of the entire lesion. Therefore, the diagnostic accuracy of CNB increases as the numbers of CNBs increases. Previous studies have reported that more than 10 samples per lesion substantially reduced the underestimation rate of ADH.(8) However, it seems almost impossible to obtain more than 10 NCB samples from each patient. Therefore, it is important to determine the predictive factors of more advanced lesions in the surgical excision specimens when ADH is diagnosed in the CNB.
This study showed that stromal alterations such as fibrosis around the ducts showing ADH occurred in the CNB, the likelihood that ductal carcinoma would be identified in the surgical excision specimens increased. Although a previous study reported that more than 4 foci of ADH and a pure micropapillary architecture were predictive factors,(7) they did not hold true this present study. In this study, there was no significance difference in the final diagnosis in excision when subgroup was made according to the number of ADH foci observed in the CNB (≤3 or ≥4, p=0.315). Therefore, the number of ADH foci in the CNB was not a predictive factor in this study, and this was thought to result from the fact that the DCIS component was not sufficiently sampled through the CNBs in many cases. The pattern of ADH in the CNBs was mostly cribriform (84.0%), and a pure micropapillary pattern was not identified. Other reports in the literature have suggested that cytologic atypia was associated with individual cell necrosis in ADH as a predictive factor,(11) but this finding was not observed in this present study. The cells forming ADH should be similar to the cells of low-grade DCIS which showed small, uniform features with rounded nuclei to define ADH.(3) Therefore, most of the cases of ADH showing cytologic atypia and individual cell necrosis were thought to actually come under the diagnosis of DCIS. When an intraductal proliferative lesion showing cytologic atypia and individual cell necrosis is identified in CNB, we believe that the term "atypical intraductal epithelial hyperplasia" or "intraductal atypia of uncertain significance" is more appropriate than the diagnosis of ADH.
This study demonstrated that the size of the largest ADH foci in CNB was not a risk factor to detect more advanced lesions in surgical excision specimens occurred (p=0.105). Previous studies have reported that the factors that affect the likelihood of observing DCIS in an excisional biopsy were the initial mammographic size of the lesion and the amount of the residual lesion after CNB, suggesting that the size of the lesion was an important parameter.(15) However, to the best of our knowledge, there has been no report that has investigated the effect of the size of the largest ADH foci in a CNB for making the diagnosis of the surgical excision specimens. It could be assumed that if the size of the largest ADH foci increased in the CNB, then the size of the entire lesion is also increased and the possibility of detecting DCIS is increased. However, there was no significant correlation between the size of the largest ADH foci in the CNB and the size of DCIS in the surgical excision specimens (p=0.097).
There was a higher probability of detecting ductal carcinoma in the surgical excision specimens when observing stromal alterations such as fibrosis and sclerosis around the ducts showing ADH (p=0.035). In general, periductal fibrosis, elastosis and inflammation can be identified in DCIS, and these features are well associated with high-grade DCIS.(16) When stromal alterations such as fibrosis and sclerosis around the ducts showing ADH were identified in the CNB, the corresponding surgical excision tissue also showed periductal stromal changes around the DCIS (Figure 1). In addition, 2 cases of IDC with extensive DCIS showed stromal reactions around both the invasive components and the surrounding DCIS. Therefore, the ADH identified in the CNB where periductal stromal alteration occurred was thought to fall under DCIS, but ADH was diagnosed according to the quantitative criteria. Stromagenesis in breast cancer can be classified into 3 stages: "normal stroma," indicating a neoplastic progression-restraining environment; "primed stroma," indicating a permissive, supportive landscape for tumor progression, and "activated stroma," indicating an advanced neoplastic microenvironment.(17) It is known that the linear progression from atypical epithelial hyperplasia to intraductal carcinoma and then to invasive carcinoma does not always apply to breast carcinogenesis.(18) In fact, the low- and high-grade DCISs were considered to be genetically distinct disorders because low-grade DCIS has shown chromosomal loss at 16q and 17p and high-grade DCIS has demonstrated chromosomal loss at 11q, 14q, 8p and 13q and chromosomal gain at 17q, 8q and 5p.(18,19) Therefore, the tumor stroma to be induced should interact with epithelial neoplastic cells that contain distinct genetic alterations.(20) This postulation could explain the finding of stromal alterations around the duct lesions in both the CNBs and the surgical excision specimens. Nine cases that showed stromal alterations around ADH in the CNB did not demonstrate ductal carcinoma in the surgical excision specimens. Out of these 9 cases, 5 were intraductal papilloma associated with sclerosis (sclerosing papilloma) and 1 was a complex sclerosing lesion. Therefore, a total of 6 cases were BPD, which could be associated with stromal change such as fibrosis and sclerosis. Further studies should be conducted to distinguish the stromal alterations in benign and malignant breast disease.
As the stromal alterations around ADH occurred in the CNB, there was an increased likelihood that more advanced lesions would be identified in the surgical excision specimens. Therefore, the stromal alterations included fibroblast proliferation, fibrosis, and sclerosis should be described in pathology report when the diagnosis of ADH is made in CNB.
Figures and Tables
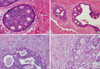 | Figure 1Histopathologic features in core needle biopsy (CNB) and subsequent surgical excision. Foci of atypical ductal hyperplasia (ADH) were identified in CNB, and there were no stromal alterations around ducts showing ADH (A, H&E stain, ×400). In subsequent surgical excision specimens, there were 2 foci of ADH (B, H&E stain, ×200). In NCB, ADH foci were noted, and there was periductal fibroblast proliferation and fibrosis in periductal stroma (C, H&E stain, ×400). In subsequent surgical excision specimens, there was ductal carcinoma in situ showing periductal fibroblast proliferation, fibrosis, and inflammatory cell infiltration (D, H&E stain, ×200). |
References
1. Bassett L, Winchester DP, Caplan RB, Dershaw DD, Dowlatshahi K, Evans WP 3rd, et al. Stereotactic core-needle biopsy of the breast: a report of the Joint Task Force of the American College of Radiology, American College of Surgeons, and College of American Pathologists. CA Cancer J Clin. 1997. 47:171–190.

2. Fisher ER, Costantino J, Fisher B, Palekar AS, Paik SM, Suarez CM, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) Protocol B-17. Five-year observations concerning lobular carcinoma in situ. Cancer. 1996. 78:1403–1416.

3. Page DL, Rogers LW. Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. Hum Pathol. 1992. 23:1095–1097.

4. Brem RF, Behrndt VS, Sanow L, Gatewood OM. Atypical ductal hyperplasia: histologic underestimation of carcinoma in tissue harvested from impalpable breast lesions using 11-gauge stereotactically guided directional vacuum-assisted biopsy. AJR Am J Roentgenol. 1999. 172:1405–1407.

5. Brown TA, Wall JW, Christensen ED, Smith DV, Holt CA, Carter PL, et al. Atypical hyperplasia in the era of stereotactic core needle biopsy. J Surg Oncol. 1998. 67:168–173.

6. Dahlstrom JE, Sutton S, Jain S. Histological precision of stereotactic core biopsy in diagnosis of malignant and premalignant breast lesions. Histopathology. 1996. 28:537–541.

7. Ely KA, Carter BA, Jensen RA, Simpson JF, Page DL. Core biopsy of the breast with atypical ductal hyperplasia: a probabilistic approach to reporting. Am J Surg Pathol. 2001. 25:1017–1021.
8. Jackman RJ, Nowels KW, Shepard MJ, Finkelstein SI, Marzoni FA Jr. Stereotaxic large-core needle biopsy of 450 nonpalpable breast lesions with surgical correlation in lesions with cancer or atypical hyperplasia. Radiology. 1994. 193:91–95.

9. Liberman L, Cohen MA, Dershaw DD, Abramson AF, Hann LE, Rosen PP. Atypical ductal hyperplasia diagnosed at stereotaxic core biopsy of breast lesions: an indication for surgical biopsy. AJR Am J Roentgenol. 1995. 164:1111–1113.

10. Moore MM, Hargett CW 3rd, Hanks JB, Fajardo LL, Harvey JA, Frierson HF Jr, et al. Association of breast cancer with the finding of atypical ductal hyperplasia at core breast biopsy. Ann Surg. 1997. 225:726–731.

11. Yeh IT, Dimitrov D, Otto P, Miller AR, Kahlenberg MS, Cruz A. Pathologic review of atypical hyperplasia identified by image-guided breast needle core biopsy. Correlation with excision specimen. Arch Pathol Lab Med. 2003. 127:49–54.

12. Tavassoli FA, Norris HJ. A comparison of the results of long-term follow-up for atypical intraductal hyperplasia and intraductal hyperplasia of the breast. Cancer. 1990. 65:518–529.

13. Silverstein MJ, Poller DN, Waisman JR, Colburn WJ, Barth A, Gierson ED, et al. Prognostic classification of breast ductal carcinoma-in-situ. Lancet. 1995. 345:1154–1157.

14. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991. 19:403–410.

15. Renshaw AA, Cartagena N, Schenkman RH, Derhagopian RP, Gould EW. Atypical ductal hyperplasia in breast core needle biopsies. Correlation of size of the lesion, complete removal of the lesion, and the incidence of carcinoma in follow-up biopsies. Am J Clin Pathol. 2001. 116:92–96.

16. Quinn CM, Ostrowski JL, Parkin GJ, Horgan K, Benson EA. Ductal carcinoma in situ of the breast: the clinical significance of histological classification. Histopathology. 1997. 30:113–119.

17. Cukierman E. A visual-quantitative analysis of fibroblastic stromagenesis in breast cancer progression. J Mammary Gland Biol Neoplasia. 2004. 9:311–324.

18. Simpson PT, Reis-Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol. 2005. 205:248–254.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download